Magnesium »
PDB 6at2-6b5e »
6b4j »
Magnesium in PDB 6b4j: Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex
Enzymatic activity of Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex
All present enzymatic activity of Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex:
3.6.4.13;
3.6.4.13;
Protein crystallography data
The structure of Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex, PDB code: 6b4j
was solved by
D.H.Lin,
A.R.Correia,
S.W.Cai,
F.M.Huber,
C.A.Jette,
A.Hoelz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.43 / 3.40 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 87.940, 73.560, 145.860, 90.00, 95.07, 90.00 |
| R / Rfree (%) | 26.1 / 31.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex
(pdb code 6b4j). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex, PDB code: 6b4j:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex, PDB code: 6b4j:
Jump to Magnesium binding site number: 1; 2;
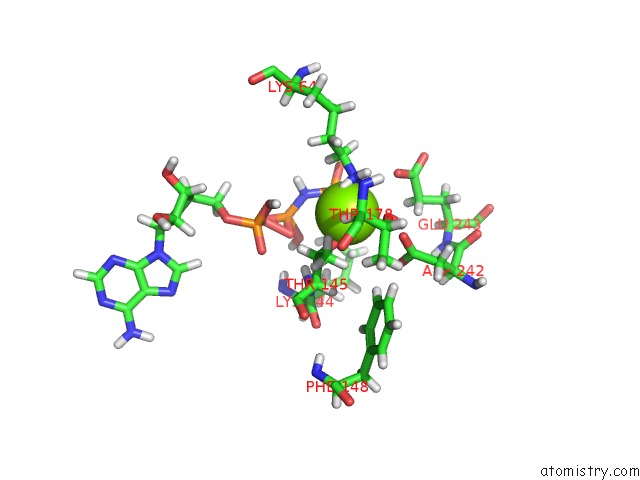
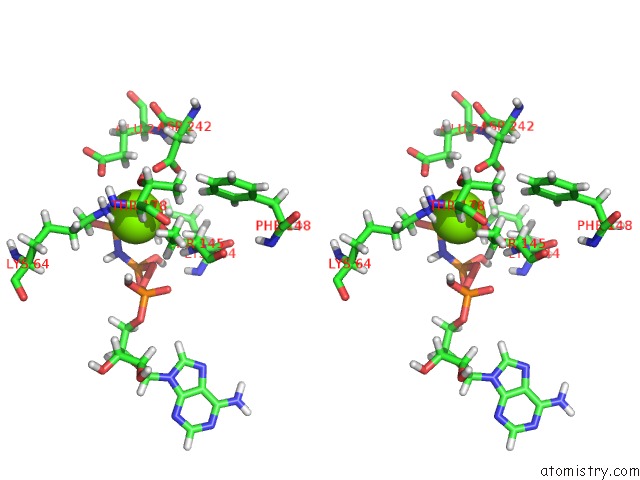
Magnesium binding site 1 out of 2 in 6b4j
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex within 5.0Å range:
|
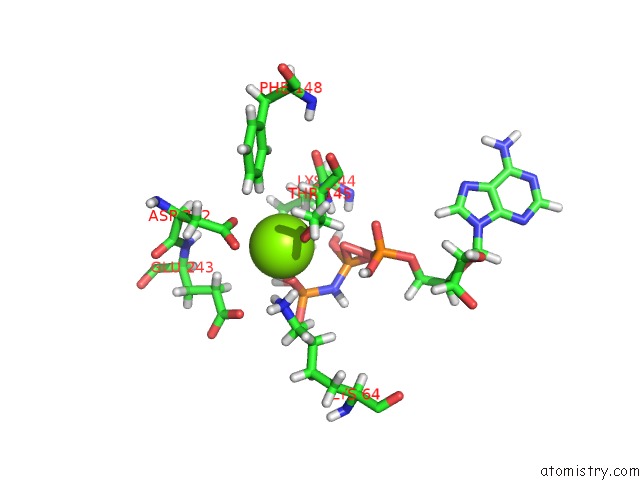
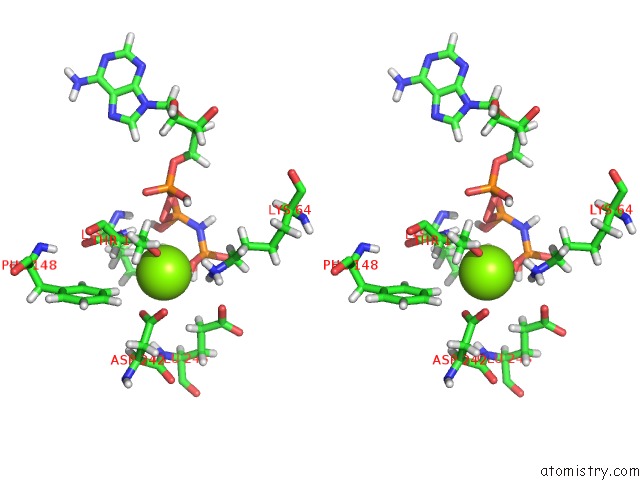
Magnesium binding site 2 out of 2 in 6b4j
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Human GLE1 Ctd-NUP42 Gbm-DDX19B(Amppnp) Complex within 5.0Å range:
|
Reference:
D.H.Lin,
A.R.Correia,
S.W.Cai,
F.M.Huber,
C.A.Jette,
A.Hoelz.
Structural and Functional Analysis of Mrna Export Regulation By the Nuclear Pore Complex. Nat Commun V. 9 2319 2018.
ISSN: ESSN 2041-1723
PubMed: 29899397
DOI: 10.1038/S41467-018-04459-3
Page generated: Wed Aug 13 02:28:11 2025
ISSN: ESSN 2041-1723
PubMed: 29899397
DOI: 10.1038/S41467-018-04459-3
Last articles
Mg in 6VCWMg in 6VCX
Mg in 6VCM
Mg in 6VCL
Mg in 6VB9
Mg in 6VC7
Mg in 6VCK
Mg in 6VC8
Mg in 6VAU
Mg in 6VAT