Magnesium »
PDB 6hdh-6hnq »
6hgq »
Magnesium in PDB 6hgq: Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
Enzymatic activity of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
All present enzymatic activity of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+:
2.4.2.7;
2.4.2.7;
Protein crystallography data
The structure of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+, PDB code: 6hgq
was solved by
P.Nioche,
J.Huyet,
M.Ozeir,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 71.69 / 1.90 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 49.060, 49.800, 71.810, 90.08, 93.22, 102.31 |
| R / Rfree (%) | 17.5 / 21.4 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
(pdb code 6hgq). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+, PDB code: 6hgq:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+, PDB code: 6hgq:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 6hgq
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
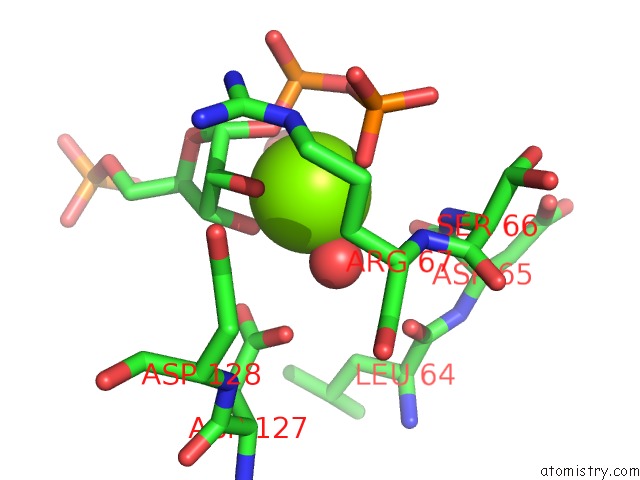
Mono view
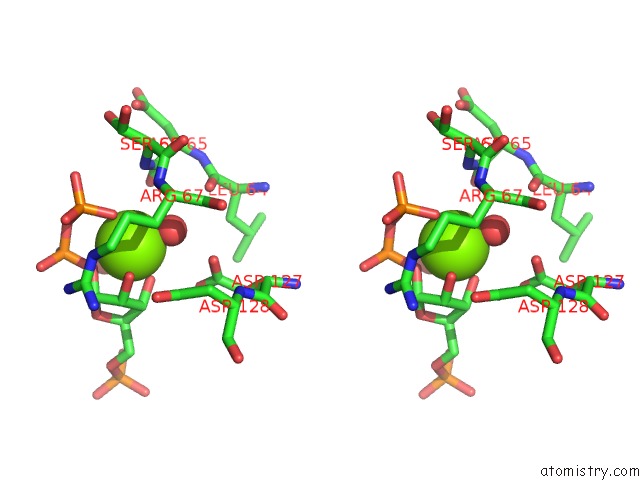
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+ within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 6hgq
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
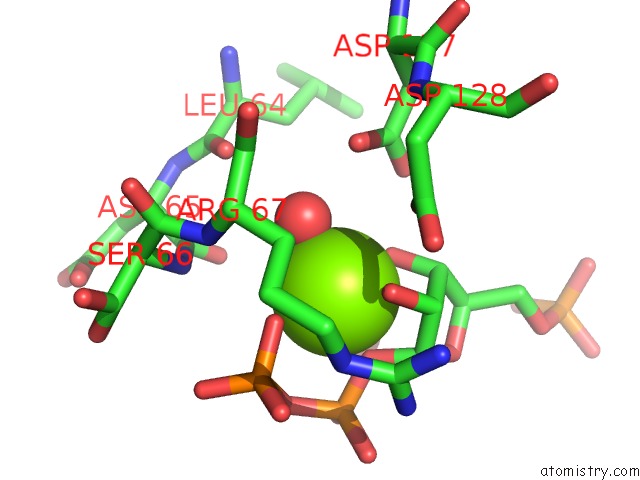
Mono view
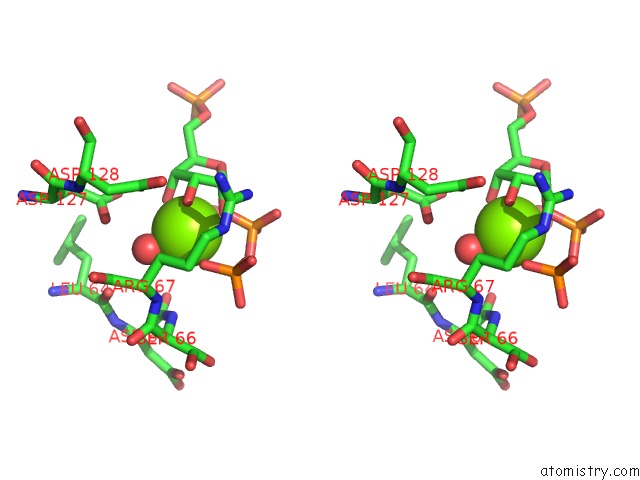
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+ within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 6hgq
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
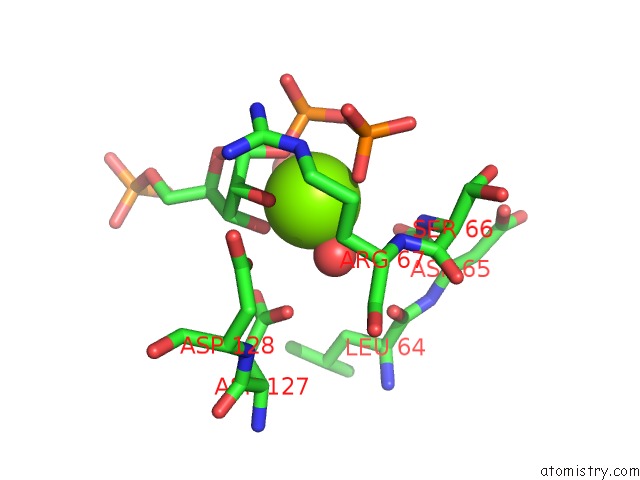
Mono view
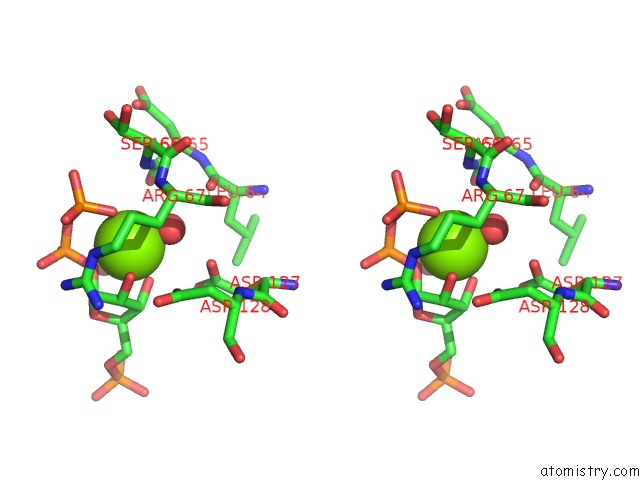
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+ within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 6hgq
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+
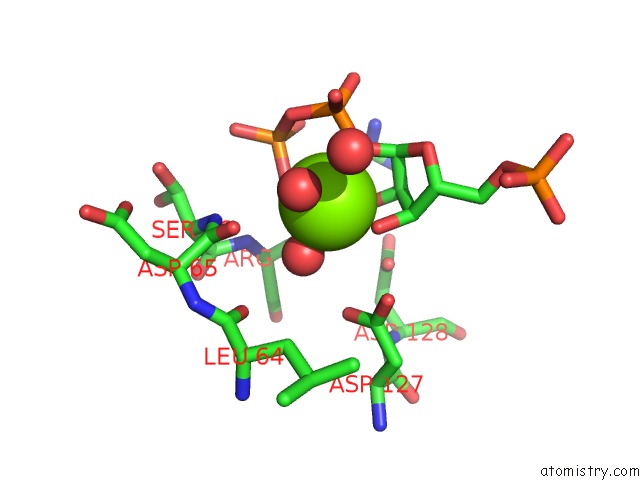
Mono view
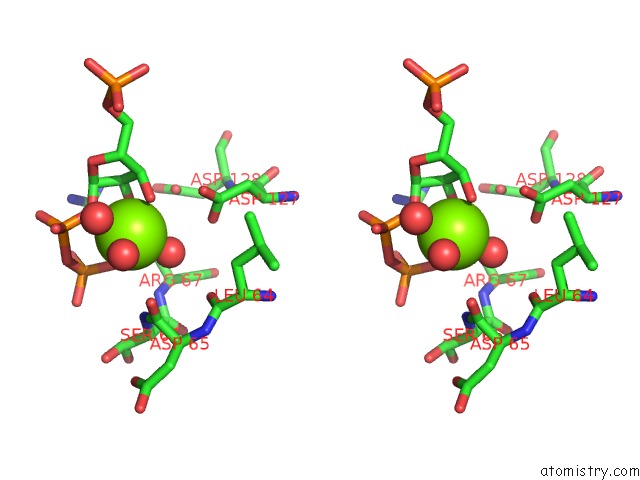
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Human Aprt Wild Type in Complex with Hypoxanthine, Prpp and MG2+ within 5.0Å range:
|
Reference:
M.Ozeir,
J.Huyet,
M.C.Burgevin,
B.Pinson,
F.Chesney,
J.M.Remy,
A.R.Siddiqi,
R.Lupoli,
G.Pinon,
C.Saint-Marc,
J.F.Gibert,
R.Morales,
I.Ceballos-Picot,
R.Barouki,
B.Daignan-Fornier,
A.Olivier-Bandini,
F.Auge,
P.Nioche.
Structural Basis For Substrate Selectivity and Nucleophilic Substitution Mechanisms in Human Adenine Phosphoribosyltransferase Catalyzed Reaction. J.Biol.Chem. V. 294 11980 2019.
ISSN: ESSN 1083-351X
PubMed: 31160323
DOI: 10.1074/JBC.RA119.009087
Page generated: Tue Oct 1 01:45:02 2024
ISSN: ESSN 1083-351X
PubMed: 31160323
DOI: 10.1074/JBC.RA119.009087
Last articles
Cl in 7SS7Cl in 7SS6
Cl in 7SQB
Cl in 7SQA
Cl in 7SPP
Cl in 7SQ3
Cl in 7SPG
Cl in 7SP3
Cl in 7SOK
Cl in 7SNN