Magnesium »
PDB 6hvw-6i0v »
6hwl »
Magnesium in PDB 6hwl: Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate
Protein crystallography data
The structure of Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate, PDB code: 6hwl
was solved by
J.A.Manso,
P.J.B.Pereira,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.89 / 2.15 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.764, 97.435, 80.188, 90.00, 107.42, 90.00 |
| R / Rfree (%) | 20.7 / 24.3 |
Other elements in 6hwl:
The structure of Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate
(pdb code 6hwl). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate, PDB code: 6hwl:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate, PDB code: 6hwl:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 6hwl
Go back to
Magnesium binding site 1 out
of 2 in the Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate
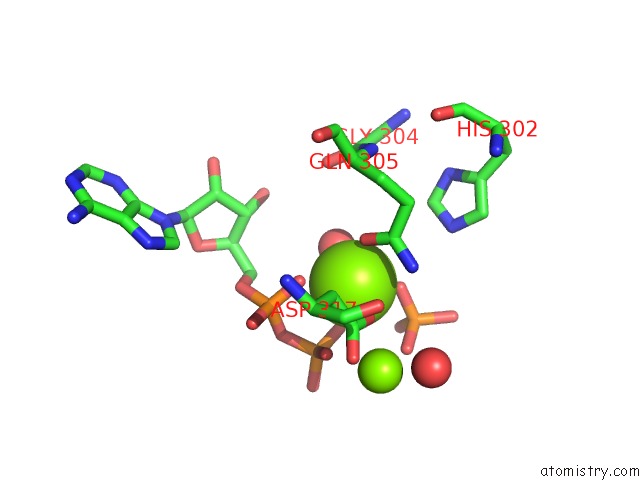
Mono view
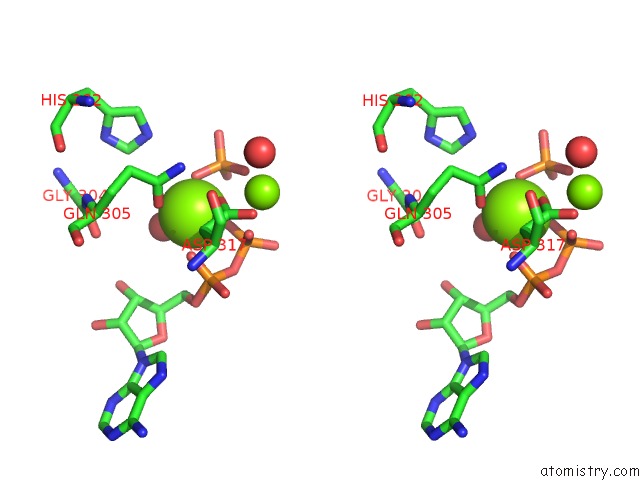
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 6hwl
Go back to
Magnesium binding site 2 out
of 2 in the Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate
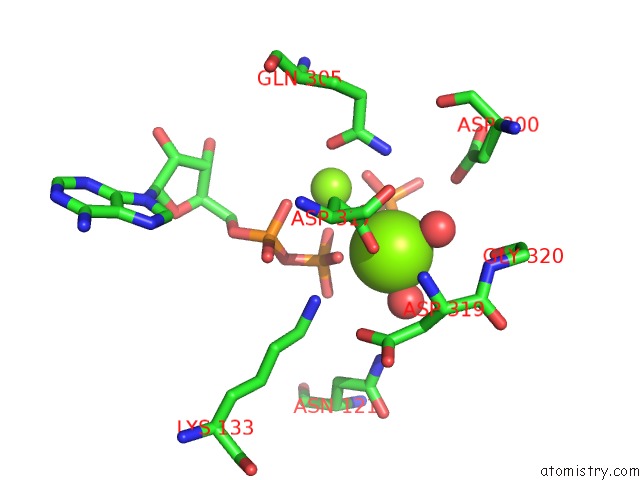
Mono view
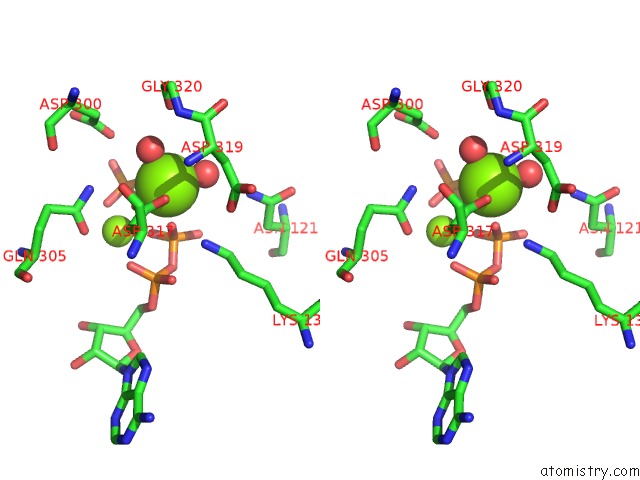
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Glucosamine Kinase in Complex with Glucosamine, Adp and Inorganic Phosphate within 5.0Å range:
|
Reference:
J.A.Manso,
D.Nunes-Costa,
S.Macedo-Ribeiro,
N.Empadinhas,
P.J.B.Pereira.
Molecular Fingerprints For A Novel Enzyme Family Inactinobacteriawith Glucosamine Kinase Activity. Mbio V. 10 2019.
ISSN: ESSN 2150-7511
PubMed: 31088917
DOI: 10.1128/MBIO.00239-19
Page generated: Tue Oct 1 02:47:11 2024
ISSN: ESSN 2150-7511
PubMed: 31088917
DOI: 10.1128/MBIO.00239-19
Last articles
Cl in 7U9VCl in 7UAH
Cl in 7U98
Cl in 7U9F
Cl in 7U6H
Cl in 7U9A
Cl in 7U99
Cl in 7U56
Cl in 7U92
Cl in 7U68