Magnesium »
PDB 6ll8-6ly7 »
6ls5 »
Magnesium in PDB 6ls5: Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor
Protein crystallography data
The structure of Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor, PDB code: 6ls5
was solved by
H.Yunyuan,
S.Rongrong,
X.Yixiang,
N.Shuaishuai,
R.Yanliang,
L.Jian,
W.Jian,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.84 / 2.03 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 67.599, 83.523, 276.929, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.4 / 23.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor
(pdb code 6ls5). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor, PDB code: 6ls5:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor, PDB code: 6ls5:
Jump to Magnesium binding site number: 1; 2; 3; 4;
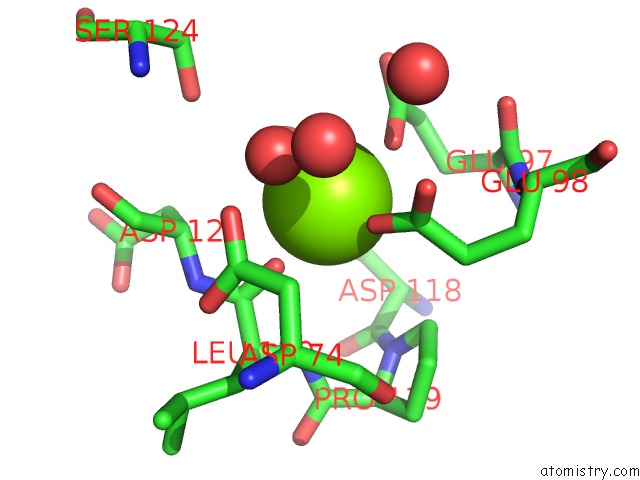
Magnesium binding site 1 out of 4 in 6ls5
Go back to
Magnesium binding site 1 out
of 4 in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 6ls5
Go back to
Magnesium binding site 2 out
of 4 in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 6ls5
Go back to
Magnesium binding site 3 out
of 4 in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor within 5.0Å range:
|
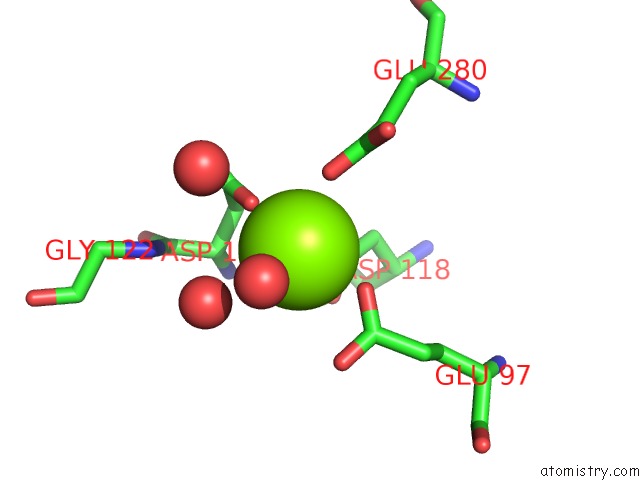
Magnesium binding site 4 out of 4 in 6ls5
Go back to
Magnesium binding site 4 out
of 4 in the Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Human Liver Fbpase Complexed with Covalent Allosteric Inhibitor within 5.0Å range:
|
Reference:
Y.Huang,
Y.Xu,
R.Song,
S.Ni,
J.Liu,
Y.Xu,
Y.Ren,
L.Rao,
Y.Wang,
L.Wei,
L.Feng,
C.Su,
C.Peng,
J.Li,
J.Wan.
Identification of the New Covalent Allosteric Binding Site of Fbpase with Disulfiram Derivatives Toward Glucose Reduction. J.Med.Chem. 2020.
ISSN: ISSN 0022-2623
PubMed: 32375478
DOI: 10.1021/ACS.JMEDCHEM.0C00699
Page generated: Wed Aug 13 11:26:54 2025
ISSN: ISSN 0022-2623
PubMed: 32375478
DOI: 10.1021/ACS.JMEDCHEM.0C00699
Last articles
Mg in 7BGIMg in 7BLX
Mg in 7BLZ
Mg in 7BOD
Mg in 7BNR
Mg in 7BNK
Mg in 7BMC
Mg in 7BM9
Mg in 7BM8
Mg in 7BM6