Magnesium »
PDB 6ojf-6oqz »
6ojr »
Magnesium in PDB 6ojr: Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
Enzymatic activity of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
All present enzymatic activity of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda:
1.13.11.43;
1.13.11.43;
Protein crystallography data
The structure of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda, PDB code: 6ojr
was solved by
E.Kuatsjah,
M.M.Verstraete,
M.J.Kobylarz,
A.K.N.Liu,
M.E.P.Murphy,
L.D.Eltis,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.27 / 2.30 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 181.364, 181.364, 94.983, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.2 / 20.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
(pdb code 6ojr). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda, PDB code: 6ojr:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda, PDB code: 6ojr:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 6ojr
Go back to
Magnesium binding site 1 out
of 3 in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
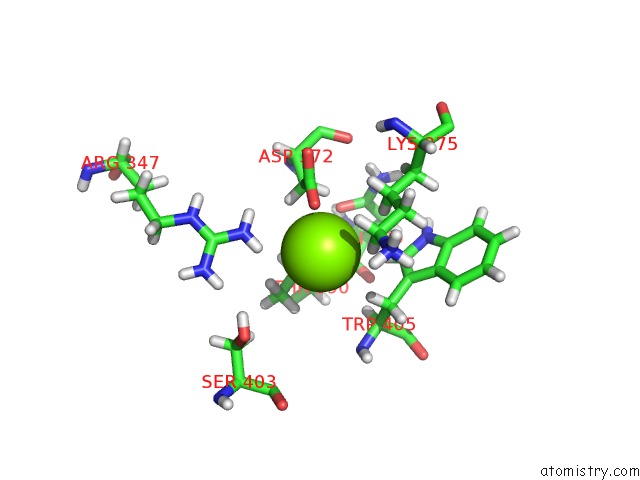
Mono view
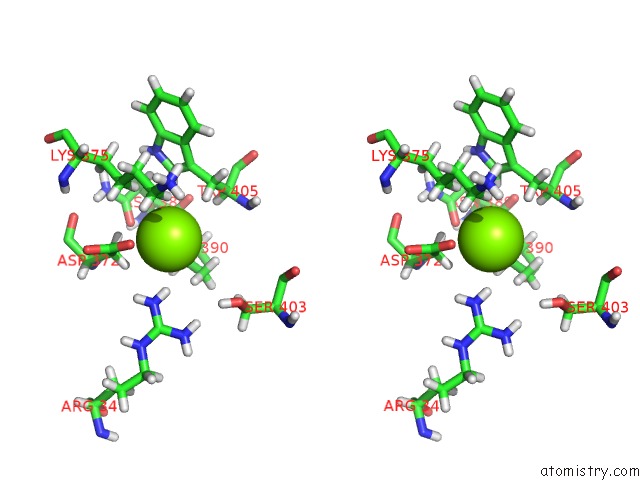
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 6ojr
Go back to
Magnesium binding site 2 out
of 3 in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
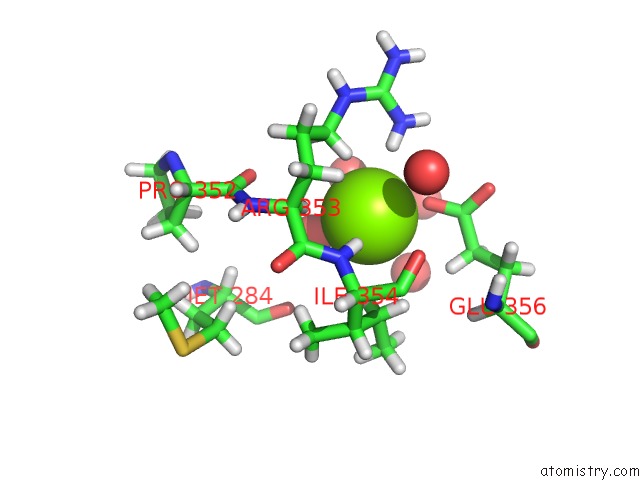
Mono view
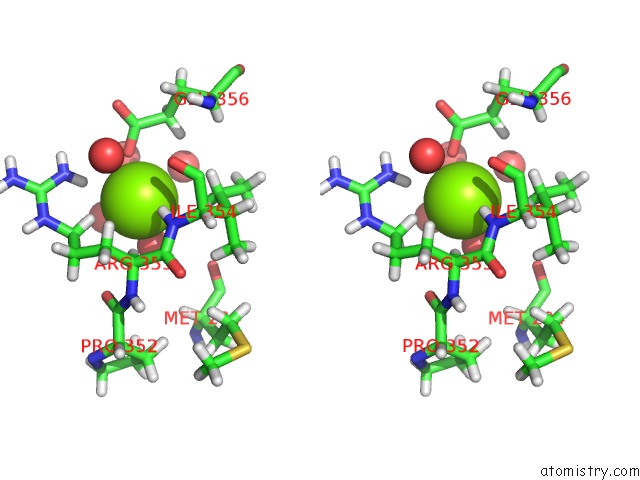
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 6ojr
Go back to
Magnesium binding site 3 out
of 3 in the Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda
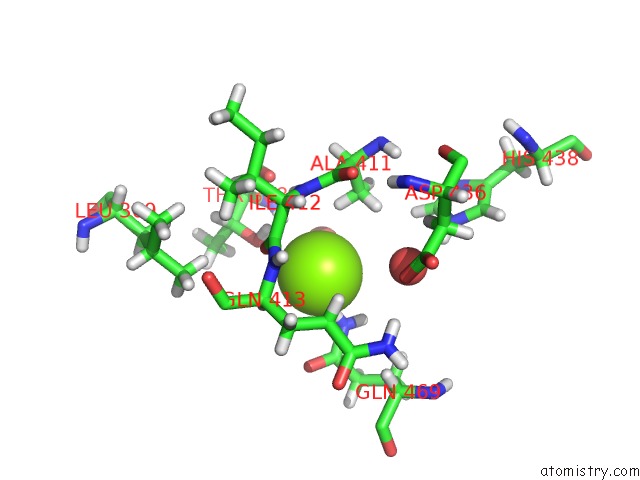
Mono view
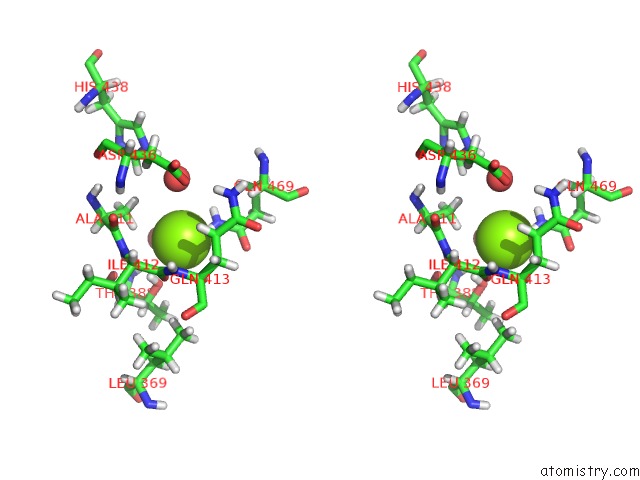
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Sphingomonas Paucimobilis TMY1009 Apo-Lsda within 5.0Å range:
|
Reference:
E.Kuatsjah,
M.M.Verstraete,
M.J.Kobylarz,
A.K.N.Liu,
M.E.P.Murphy,
L.D.Eltis.
Identification of Functionally Important Residues and Structural Features in A Bacterial Lignostilbene Dioxygenase. J.Biol.Chem. V. 294 12911 2019.
ISSN: ESSN 1083-351X
PubMed: 31292192
DOI: 10.1074/JBC.RA119.009428
Page generated: Tue Oct 1 13:24:19 2024
ISSN: ESSN 1083-351X
PubMed: 31292192
DOI: 10.1074/JBC.RA119.009428
Last articles
F in 4IVOF in 4IV4
F in 4IVM
F in 4IV2
F in 4IUI
F in 4IN4
F in 4IU7
F in 4ITI
F in 4IUE
F in 4IRU