Magnesium »
PDB 6ojf-6oqz »
6on2 »
Magnesium in PDB 6on2: Lon Protease From Yersinia Pestis with Y2853 Substrate
Enzymatic activity of Lon Protease From Yersinia Pestis with Y2853 Substrate
All present enzymatic activity of Lon Protease From Yersinia Pestis with Y2853 Substrate:
3.4.21.53;
3.4.21.53;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Lon Protease From Yersinia Pestis with Y2853 Substrate
(pdb code 6on2). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 5 binding sites of Magnesium where determined in the Lon Protease From Yersinia Pestis with Y2853 Substrate, PDB code: 6on2:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Magnesium where determined in the Lon Protease From Yersinia Pestis with Y2853 Substrate, PDB code: 6on2:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
Magnesium binding site 1 out of 5 in 6on2
Go back to
Magnesium binding site 1 out
of 5 in the Lon Protease From Yersinia Pestis with Y2853 Substrate
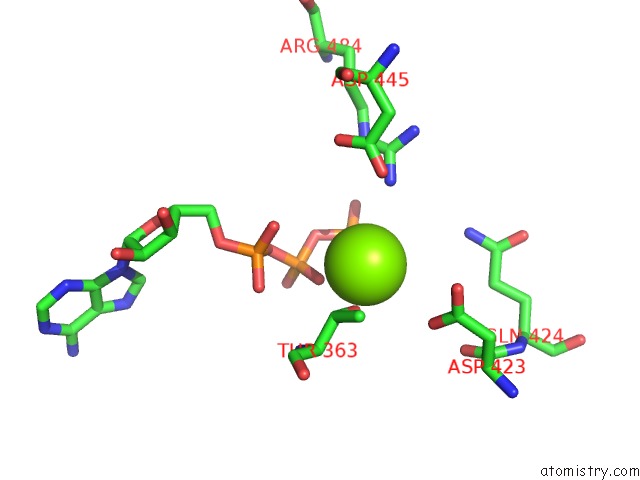
Mono view
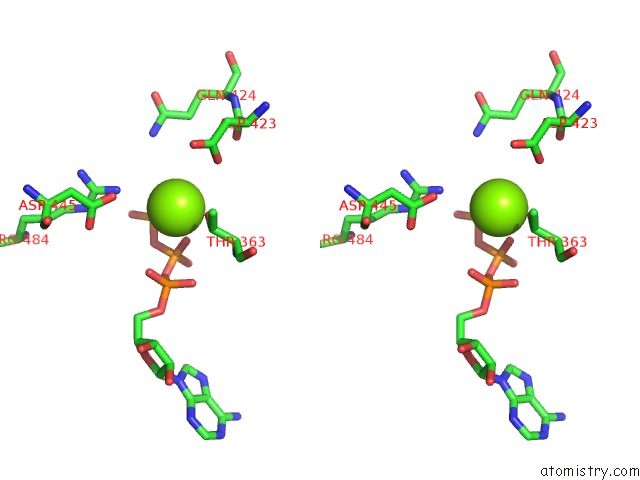
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Lon Protease From Yersinia Pestis with Y2853 Substrate within 5.0Å range:
|
Magnesium binding site 2 out of 5 in 6on2
Go back to
Magnesium binding site 2 out
of 5 in the Lon Protease From Yersinia Pestis with Y2853 Substrate
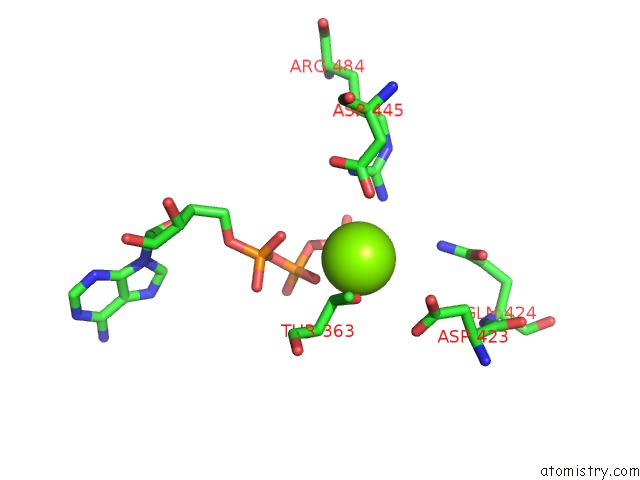
Mono view
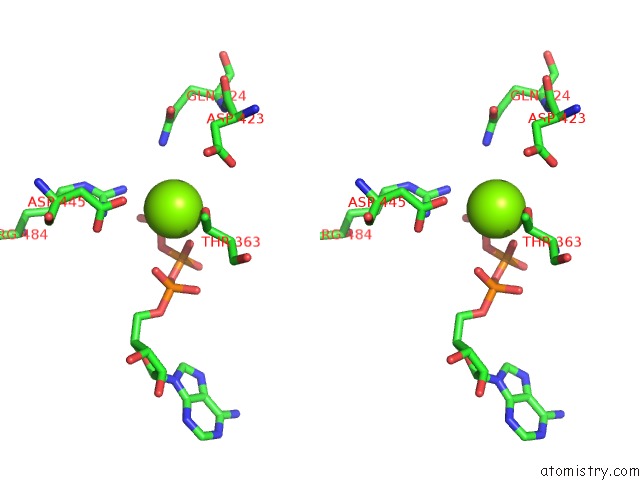
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Lon Protease From Yersinia Pestis with Y2853 Substrate within 5.0Å range:
|
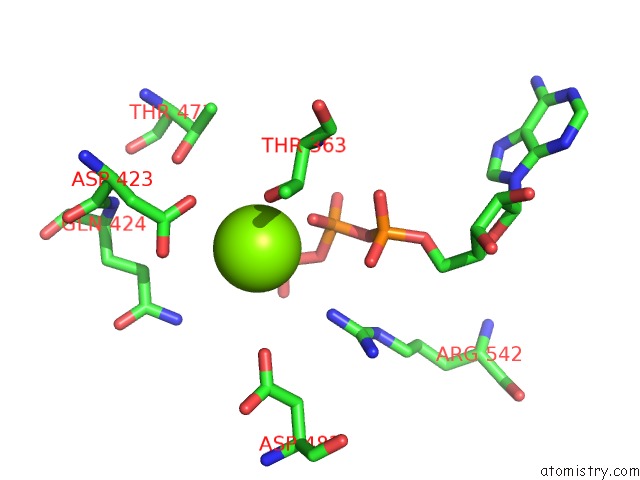
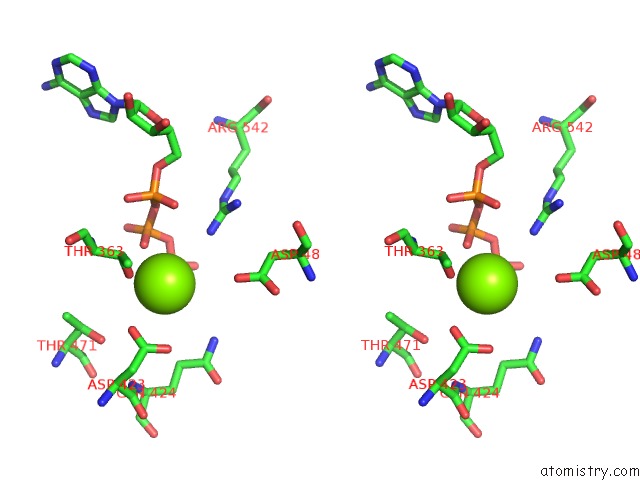
Magnesium binding site 3 out of 5 in 6on2
Go back to
Magnesium binding site 3 out
of 5 in the Lon Protease From Yersinia Pestis with Y2853 Substrate
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Lon Protease From Yersinia Pestis with Y2853 Substrate within 5.0Å range:
|
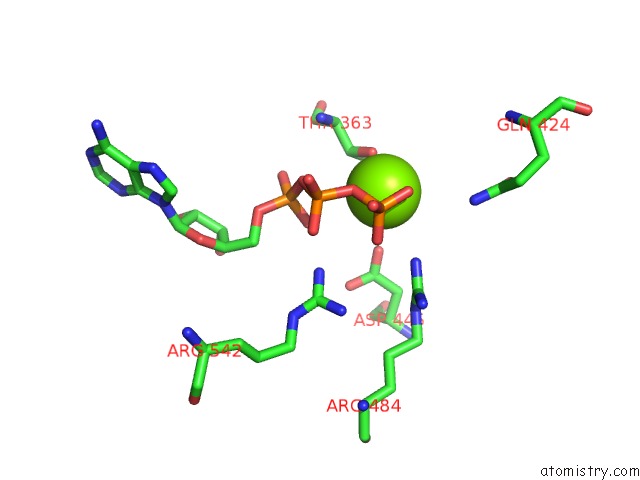
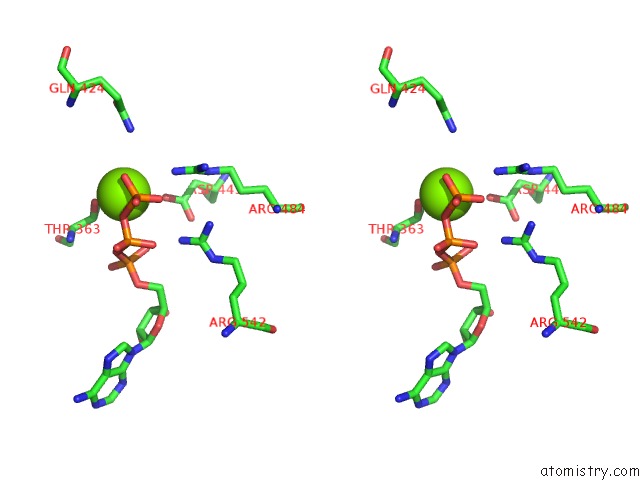
Magnesium binding site 4 out of 5 in 6on2
Go back to
Magnesium binding site 4 out
of 5 in the Lon Protease From Yersinia Pestis with Y2853 Substrate
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Lon Protease From Yersinia Pestis with Y2853 Substrate within 5.0Å range:
|
Magnesium binding site 5 out of 5 in 6on2
Go back to
Magnesium binding site 5 out
of 5 in the Lon Protease From Yersinia Pestis with Y2853 Substrate
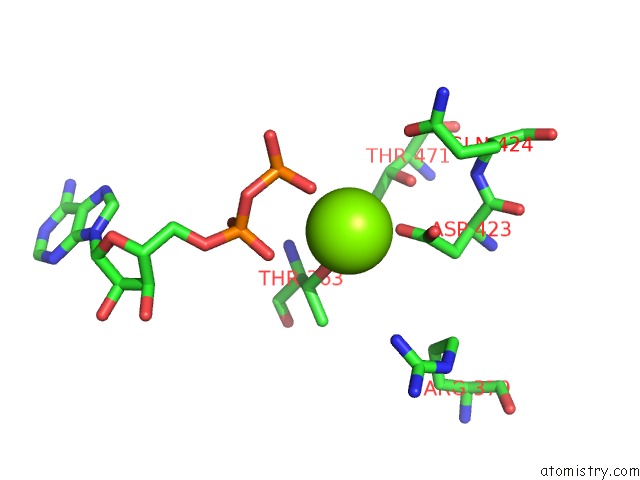
Mono view
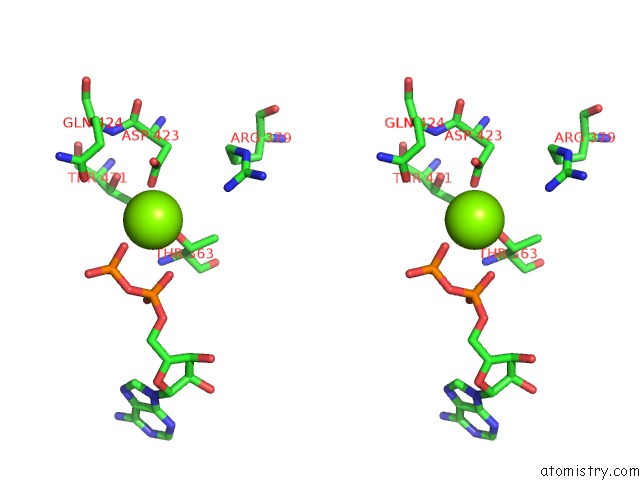
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Lon Protease From Yersinia Pestis with Y2853 Substrate within 5.0Å range:
|
Reference:
M.Shin,
A.Asmita,
C.Puchades,
E.Adjei,
R.L.Wiseman,
A.W.Karzai,
G.C.Lander.
Distinct Structural Features of the Lon Protease Drive Conserved Hand-Over-Hand Substrate Translocation To Be Published.
Page generated: Tue Oct 1 13:27:22 2024
Last articles
Cl in 5ZEQCl in 5ZDL
Cl in 5ZDK
Cl in 5ZDO
Cl in 5ZC7
Cl in 5ZAX
Cl in 5ZB4
Cl in 5ZBX
Cl in 5Z9B
Cl in 5ZB0