Magnesium »
PDB 6peu-6psq »
6peu »
Magnesium in PDB 6peu: Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
Protein crystallography data
The structure of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide, PDB code: 6peu
was solved by
S.-H.Dong,
S.K.Nair,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.00 / 1.95 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.324, 149.755, 105.671, 90.00, 103.57, 90.00 |
| R / Rfree (%) | 19.5 / 23.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
(pdb code 6peu). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 7 binding sites of Magnesium where determined in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide, PDB code: 6peu:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Magnesium where determined in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide, PDB code: 6peu:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
Magnesium binding site 1 out of 7 in 6peu
Go back to
Magnesium binding site 1 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
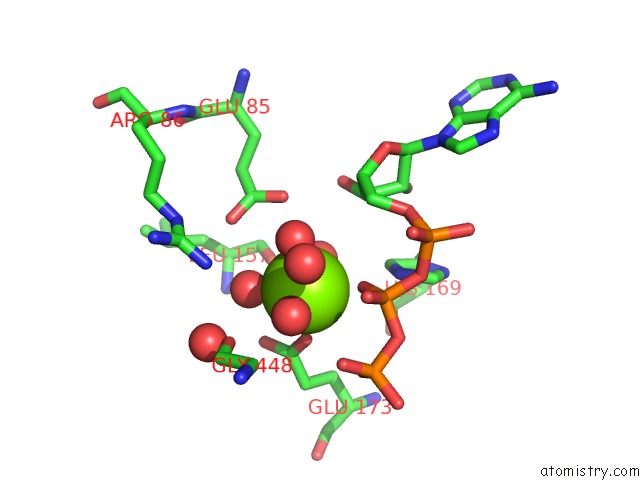
Mono view
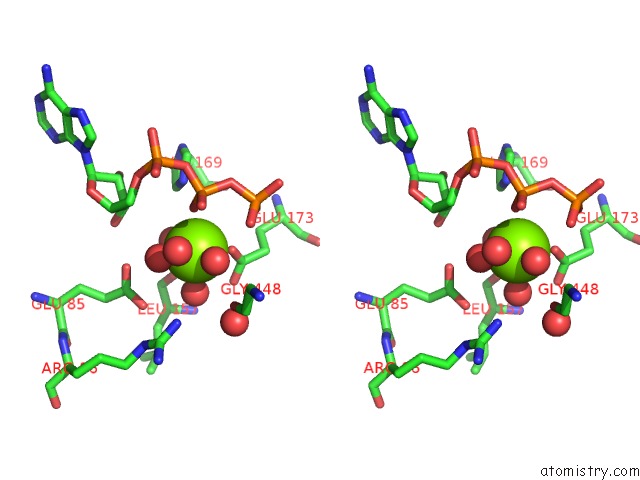
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 2 out of 7 in 6peu
Go back to
Magnesium binding site 2 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
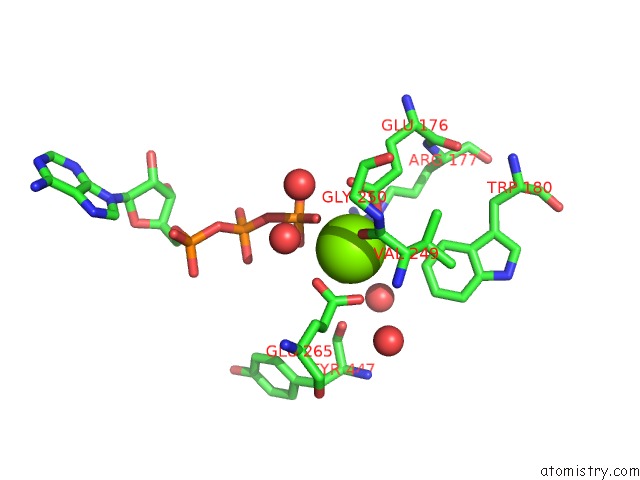
Mono view
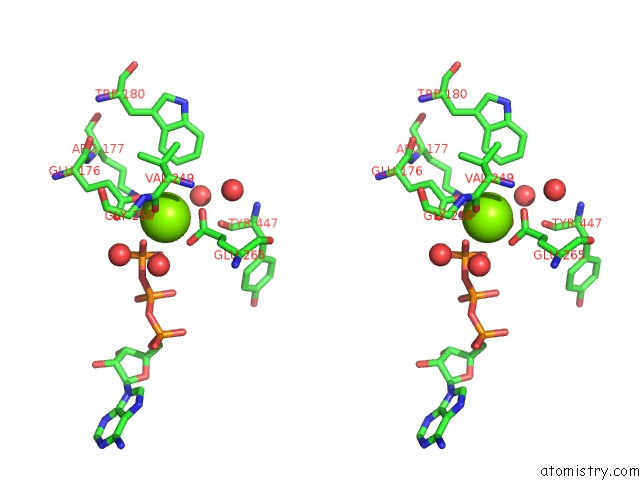
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 3 out of 7 in 6peu
Go back to
Magnesium binding site 3 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
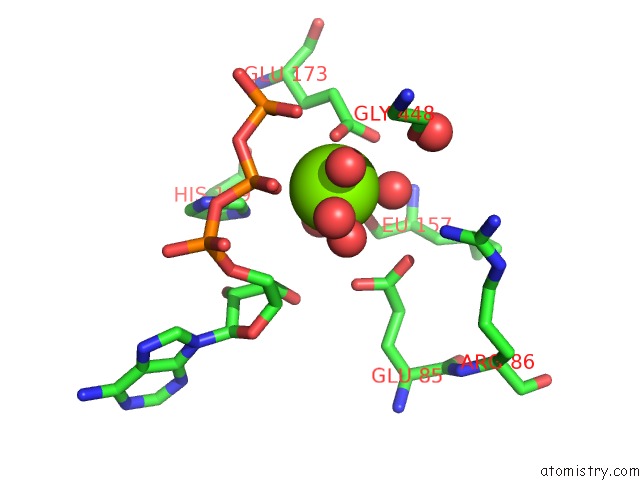
Mono view
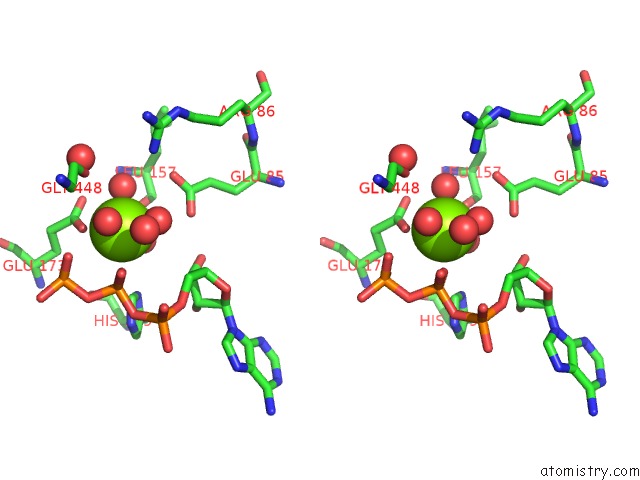
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 4 out of 7 in 6peu
Go back to
Magnesium binding site 4 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
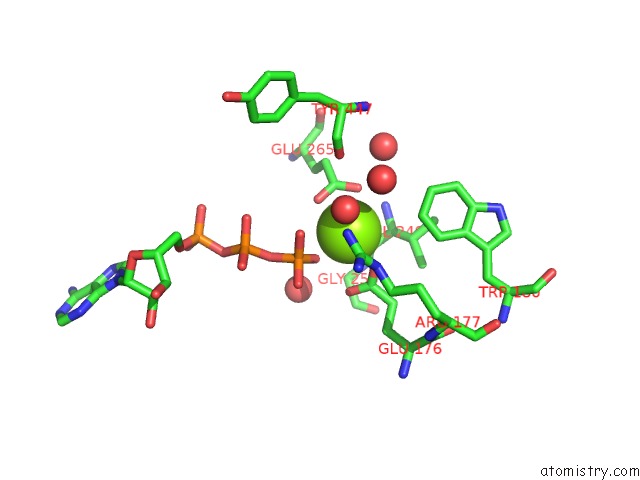
Mono view
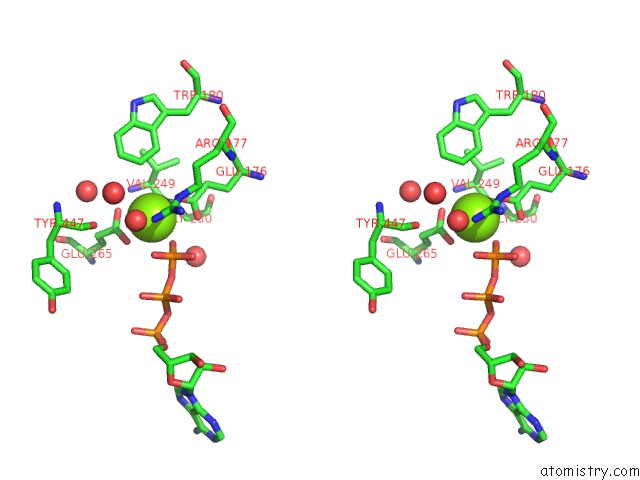
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 5 out of 7 in 6peu
Go back to
Magnesium binding site 5 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
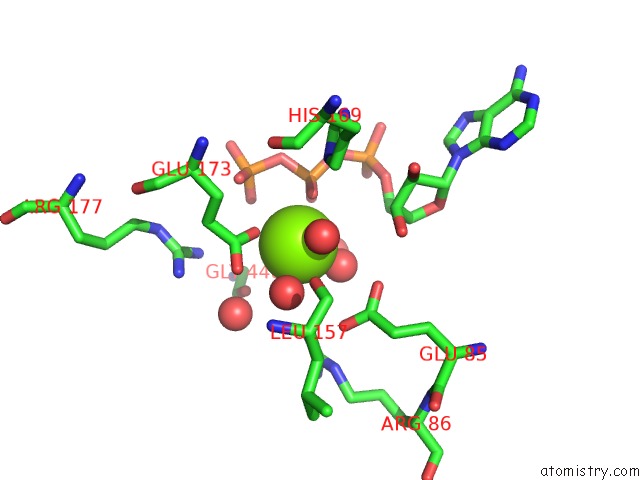
Mono view
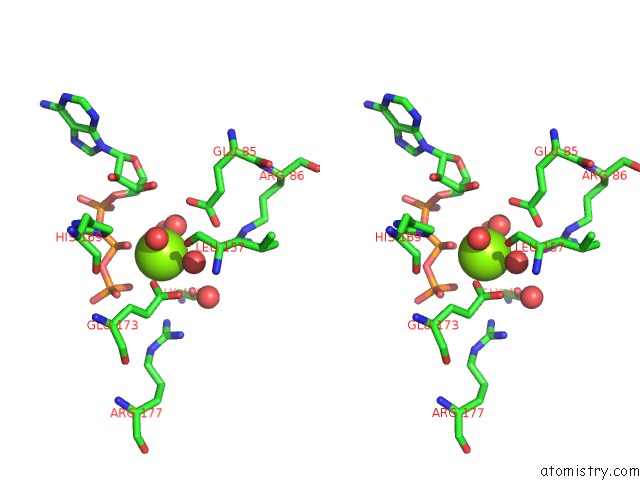
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 6 out of 7 in 6peu
Go back to
Magnesium binding site 6 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
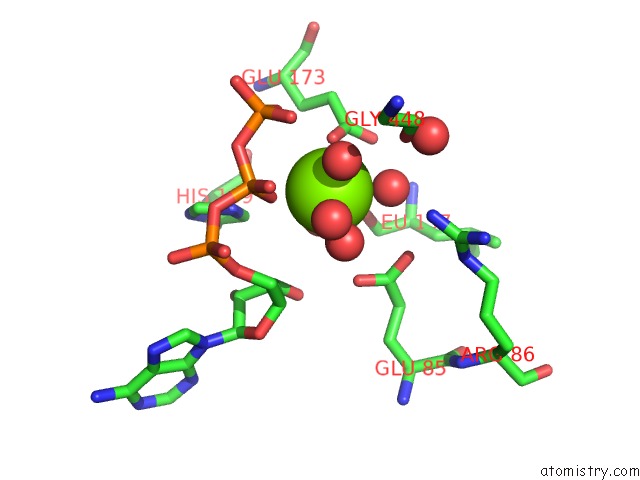
Mono view
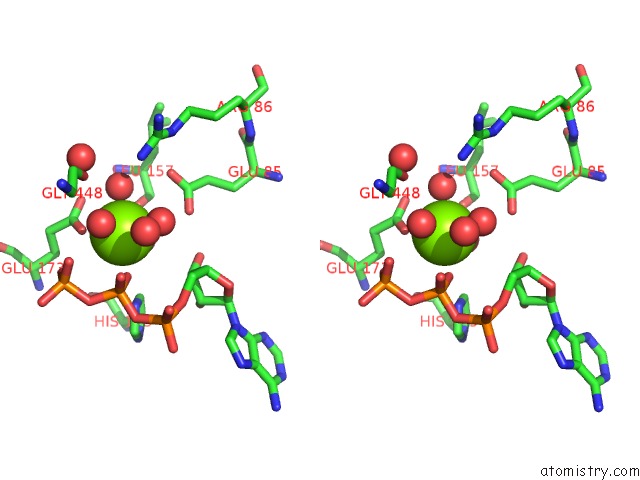
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Magnesium binding site 7 out of 7 in 6peu
Go back to
Magnesium binding site 7 out
of 7 in the Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide
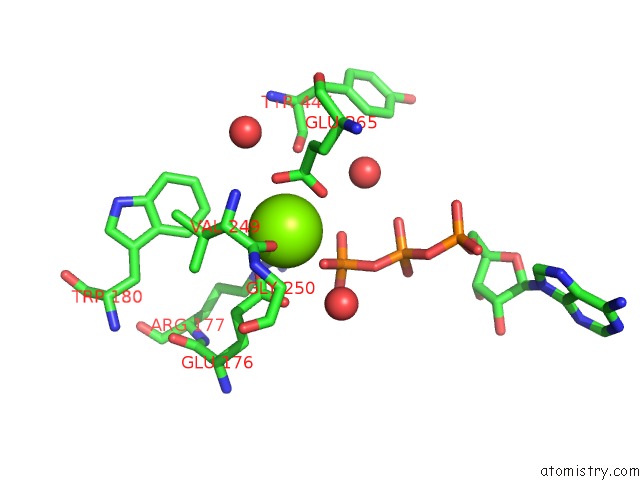
Mono view
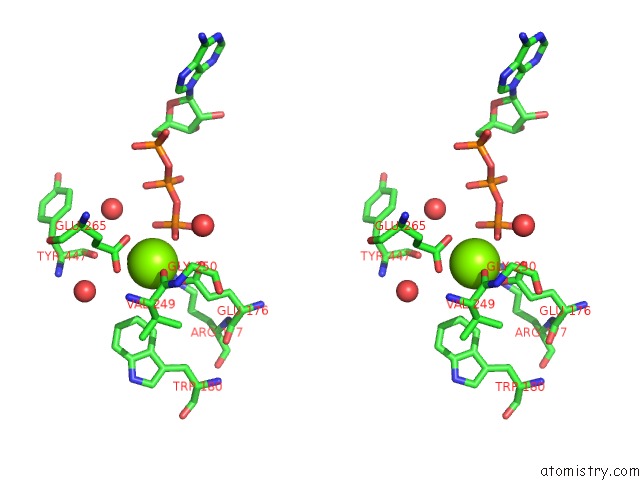
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Structure of Ycao Enzyme From Methanocaldococcus Jannaschii in Complex with Peptide within 5.0Å range:
|
Reference:
S.H.Dong,
A.Liu,
N.Mahanta,
D.A.Mitchell,
S.K.Nair.
Mechanistic Basis For Ribosomal Peptide Backbone Modifications. Acs Cent.Sci. V. 5 842 2019.
ISSN: ESSN 2374-7951
PubMed: 31139720
DOI: 10.1021/ACSCENTSCI.9B00124
Page generated: Tue Oct 1 14:07:21 2024
ISSN: ESSN 2374-7951
PubMed: 31139720
DOI: 10.1021/ACSCENTSCI.9B00124
Last articles
Cl in 5W15Cl in 5W4M
Cl in 5W4I
Cl in 5W4L
Cl in 5W44
Cl in 5W4D
Cl in 5W2O
Cl in 5W2J
Cl in 5VZH
Cl in 5VZB