Magnesium »
PDB 6ufj-6up4 »
6umr »
Magnesium in PDB 6umr: Structure of DUF89 - D291A Mutant
Protein crystallography data
The structure of Structure of DUF89 - D291A Mutant, PDB code: 6umr
was solved by
J.J.Perry,
N.Kenjic,
T.N.Dennis,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.49 / 2.21 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 91.260, 194.691, 114.940, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.1 / 25.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of DUF89 - D291A Mutant
(pdb code 6umr). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of DUF89 - D291A Mutant, PDB code: 6umr:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of DUF89 - D291A Mutant, PDB code: 6umr:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 6umr
Go back to
Magnesium binding site 1 out
of 2 in the Structure of DUF89 - D291A Mutant
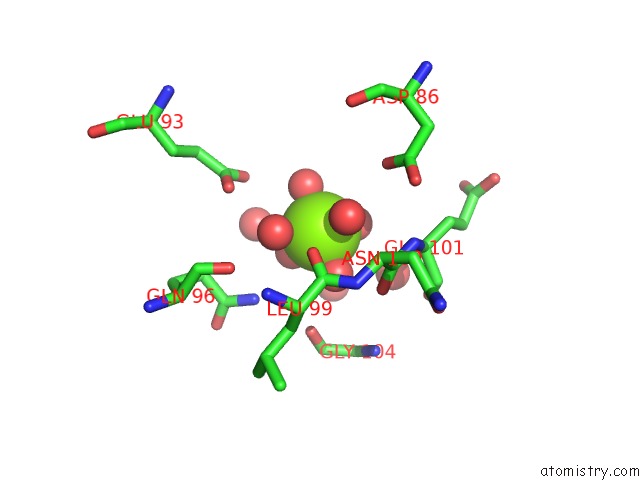
Mono view
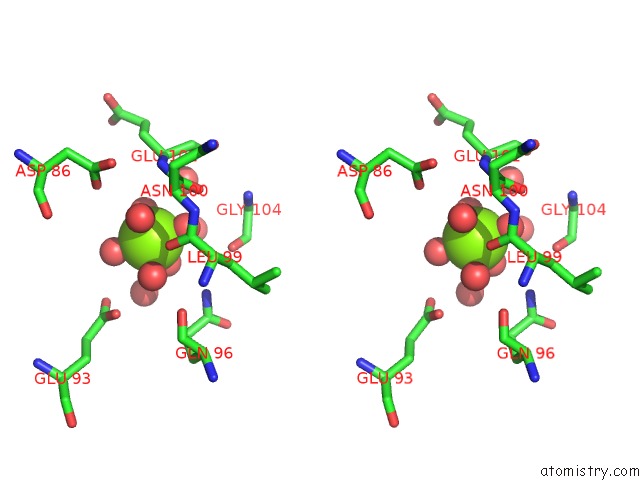
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of DUF89 - D291A Mutant within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 6umr
Go back to
Magnesium binding site 2 out
of 2 in the Structure of DUF89 - D291A Mutant
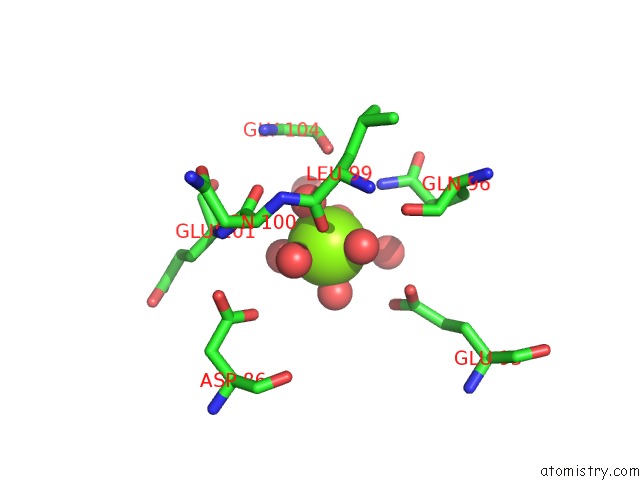
Mono view
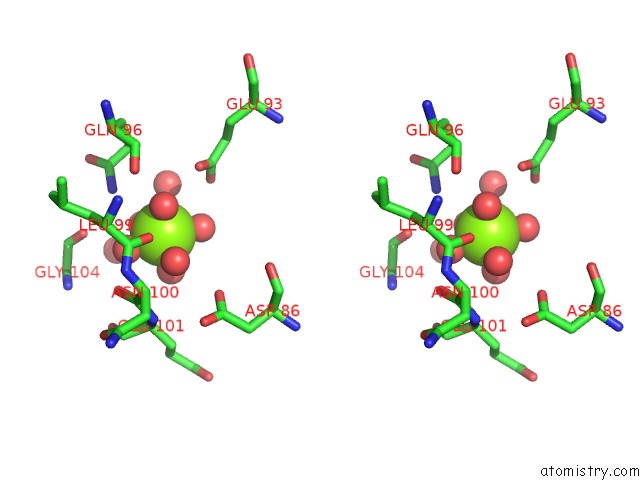
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of DUF89 - D291A Mutant within 5.0Å range:
|
Reference:
T.N.Dennis,
N.Kenjic,
A.S.Kang,
J.D.Lowenson,
J.S.Kirkwood,
S.G.Clarke,
J.J.P.Perry.
Human ARMT1 Structure and Substrate Specificity Indicates That It Is A DUF89 Family Damage-Control Phosphatase. J.Struct.Biol. V. 212 07576 2020.
ISSN: ESSN 1095-8657
PubMed: 32682077
DOI: 10.1016/J.JSB.2020.107576
Page generated: Tue Oct 1 21:07:35 2024
ISSN: ESSN 1095-8657
PubMed: 32682077
DOI: 10.1016/J.JSB.2020.107576
Last articles
Cl in 7TN0Cl in 7TNO
Cl in 7TNU
Cl in 7TNM
Cl in 7TNN
Cl in 7TNL
Cl in 7TNK
Cl in 7TNJ
Cl in 7TNH
Cl in 7TNF