Magnesium »
PDB 6zup-7a1x »
7a19 »
Magnesium in PDB 7a19: 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae
Protein crystallography data
The structure of 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae, PDB code: 7a19
was solved by
G.Hofer,
W.Keller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.64 / 1.21 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 99.794, 99.794, 129.177, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.1 / 17.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae
(pdb code 7a19). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae, PDB code: 7a19:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae, PDB code: 7a19:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 7a19
Go back to
Magnesium binding site 1 out
of 3 in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 7a19
Go back to
Magnesium binding site 2 out
of 3 in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae
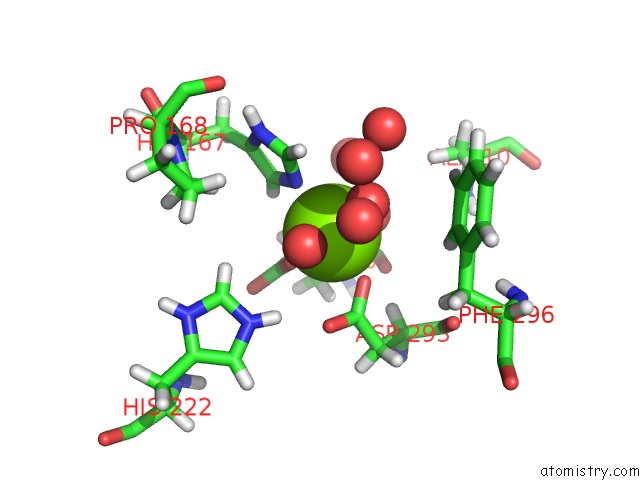
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 7a19
Go back to
Magnesium binding site 3 out
of 3 in the 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of 2,3-Dihydroxybenzoate Decarboxylase of Aspergillus Oryzae within 5.0Å range:
|
Reference:
G.Hofer,
X.Sheng,
S.Braeuer,
S.E.Payer,
K.Plasch,
W.Goessler,
K.Faber,
W.Keller,
F.Himo,
S.M.Glueck.
Metal Ion Promiscuity and Structure of 2,3-Dihydroxybenzoic Acid Decarboxylase of Aspergillus Oryzae. Chembiochem 2020.
ISSN: ESSN 1439-7633
PubMed: 33090643
DOI: 10.1002/CBIC.202000600
Page generated: Wed Oct 2 04:29:39 2024
ISSN: ESSN 1439-7633
PubMed: 33090643
DOI: 10.1002/CBIC.202000600
Last articles
Cl in 7TE2Cl in 7TEK
Cl in 7TCY
Cl in 7TCT
Cl in 7TBV
Cl in 7T8Q
Cl in 7TCD
Cl in 7TB0
Cl in 7TBS
Cl in 7TBQ