Magnesium »
PDB 7c7j-7cji »
7c91 »
Magnesium in PDB 7c91: Blasnase-T13A with D-Asn
Protein crystallography data
The structure of Blasnase-T13A with D-Asn, PDB code: 7c91
was solved by
F.Lu,
T.Ran,
L.Jiao,
W.Wang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 72.25 / 1.98 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 92.26, 92.26, 231.46, 90, 90, 90 |
| R / Rfree (%) | 18.3 / 21.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Blasnase-T13A with D-Asn
(pdb code 7c91). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 5 binding sites of Magnesium where determined in the Blasnase-T13A with D-Asn, PDB code: 7c91:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Magnesium where determined in the Blasnase-T13A with D-Asn, PDB code: 7c91:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
Magnesium binding site 1 out of 5 in 7c91
Go back to
Magnesium binding site 1 out
of 5 in the Blasnase-T13A with D-Asn
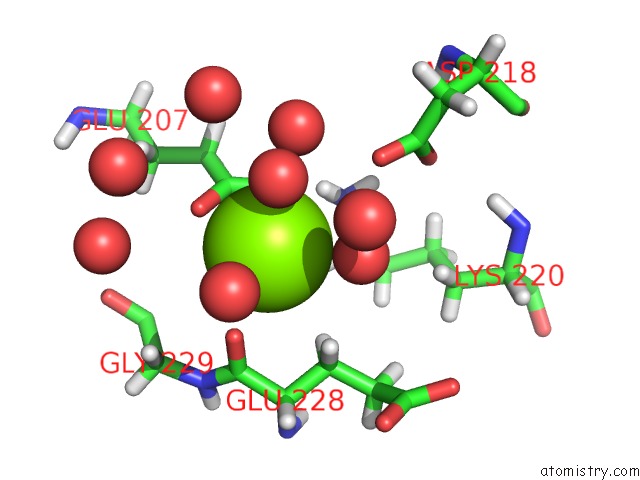
Mono view
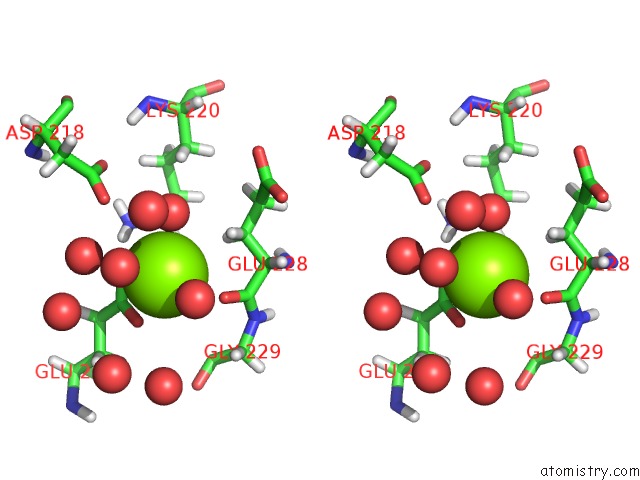
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Blasnase-T13A with D-Asn within 5.0Å range:
|
Magnesium binding site 2 out of 5 in 7c91
Go back to
Magnesium binding site 2 out
of 5 in the Blasnase-T13A with D-Asn
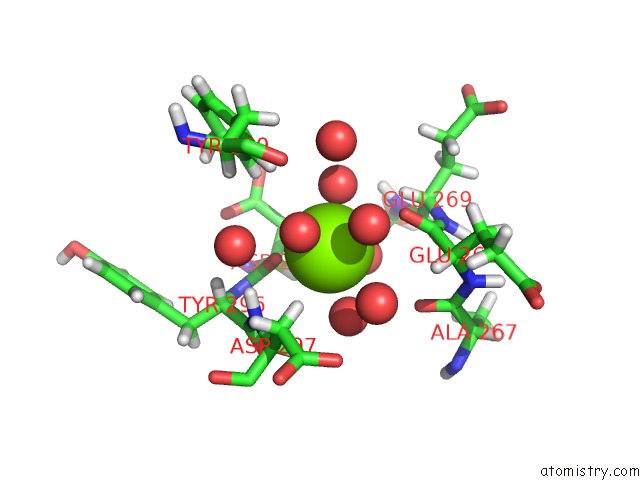
Mono view
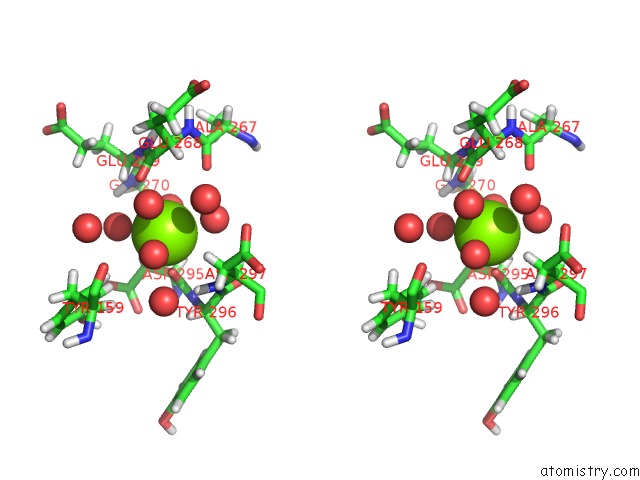
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Blasnase-T13A with D-Asn within 5.0Å range:
|
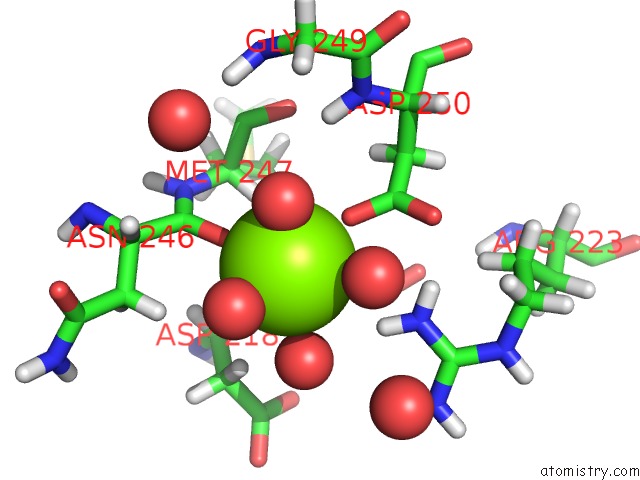
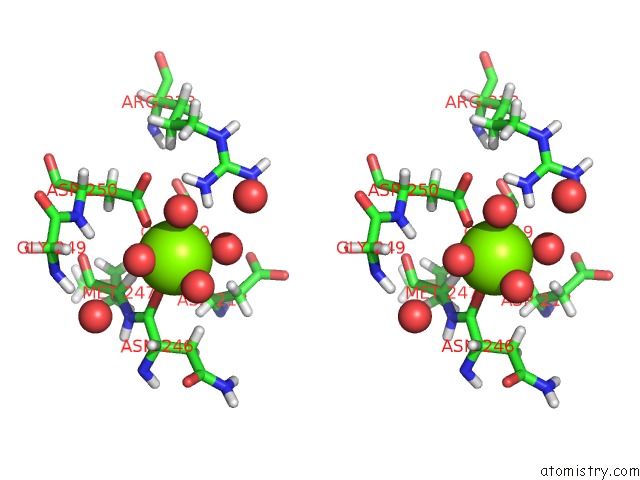
Magnesium binding site 3 out of 5 in 7c91
Go back to
Magnesium binding site 3 out
of 5 in the Blasnase-T13A with D-Asn
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Blasnase-T13A with D-Asn within 5.0Å range:
|
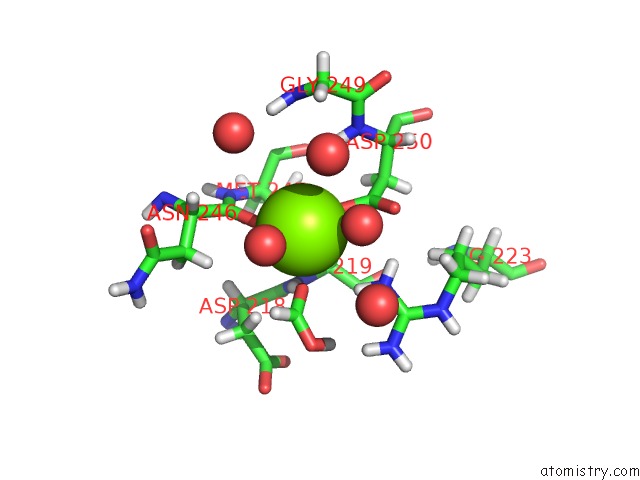
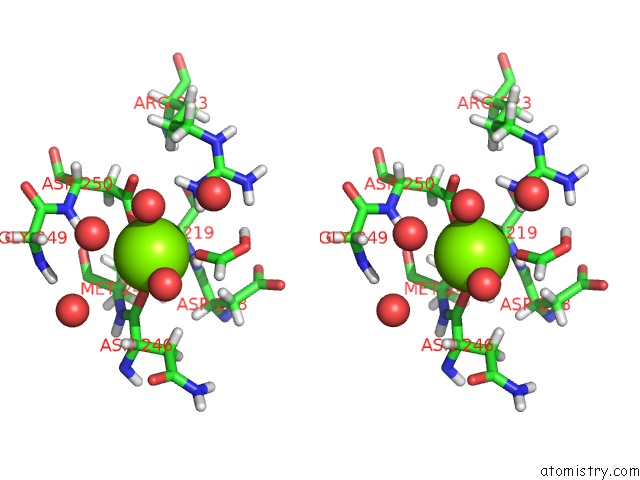
Magnesium binding site 4 out of 5 in 7c91
Go back to
Magnesium binding site 4 out
of 5 in the Blasnase-T13A with D-Asn
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Blasnase-T13A with D-Asn within 5.0Å range:
|
Magnesium binding site 5 out of 5 in 7c91
Go back to
Magnesium binding site 5 out
of 5 in the Blasnase-T13A with D-Asn
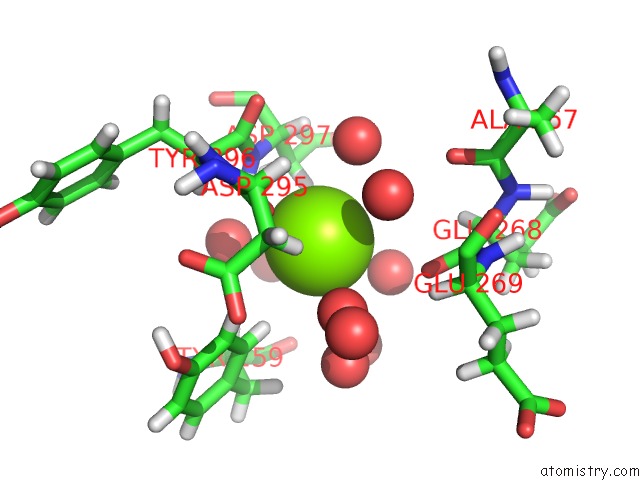
Mono view
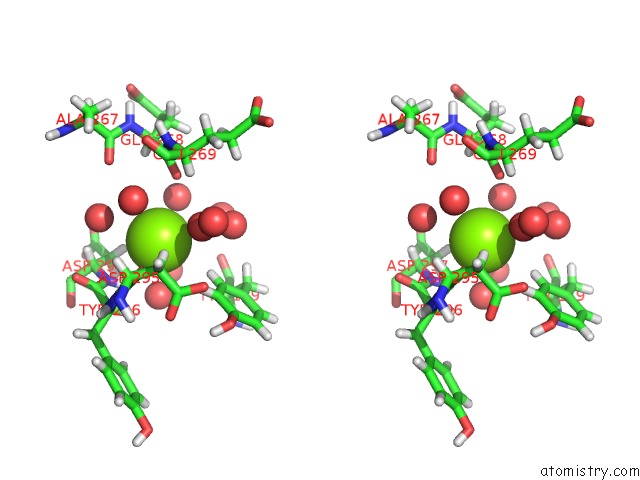
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Blasnase-T13A with D-Asn within 5.0Å range:
|
Reference:
T.Ran,
L.Jiao,
W.Wang,
J.Chen,
H.Chi,
Z.Lu,
C.Zhang,
D.Xu,
F.Lu.
Structures of L-Asparaginase From Bacillus Licheniformis Reveal An Essential Residue For Its Substrate Stereoselectivity. J.Agric.Food Chem. V. 69 223 2021.
ISSN: ESSN 1520-5118
PubMed: 33371681
DOI: 10.1021/ACS.JAFC.0C06609
Page generated: Wed Oct 2 13:53:48 2024
ISSN: ESSN 1520-5118
PubMed: 33371681
DOI: 10.1021/ACS.JAFC.0C06609
Last articles
F in 4JPSF in 4JNC
F in 4JMA
F in 4JOA
F in 4JLN
F in 4JLT
F in 4JLM
F in 4JLG
F in 4JJU
F in 4JLK