Magnesium »
PDB 7diy-7dui »
7dpj »
Magnesium in PDB 7dpj: H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control
Enzymatic activity of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control
All present enzymatic activity of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control:
3.6.5.2;
3.6.5.2;
Protein crystallography data
The structure of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control, PDB code: 7dpj
was solved by
H.Taniguchi,
S.Matsumoto,
R.Miyamoto,
T.Kawamura,
T.Kumasaka,
T.Kataoka,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.93 / 1.98 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 91.844, 91.844, 121.413, 90, 90, 120 |
| R / Rfree (%) | 19 / 22.1 |
Other elements in 7dpj:
The structure of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control also contains other interesting chemical elements:
| Calcium | (Ca) | 3 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control
(pdb code 7dpj). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control, PDB code: 7dpj:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control, PDB code: 7dpj:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 7dpj
Go back to
Magnesium binding site 1 out
of 2 in the H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control
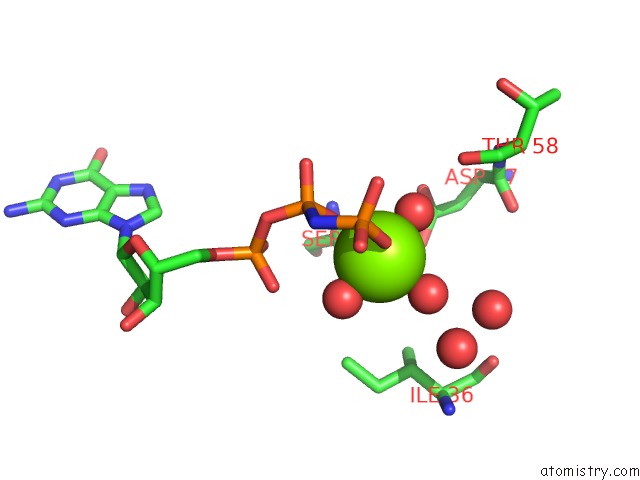
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 7dpj
Go back to
Magnesium binding site 2 out
of 2 in the H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control
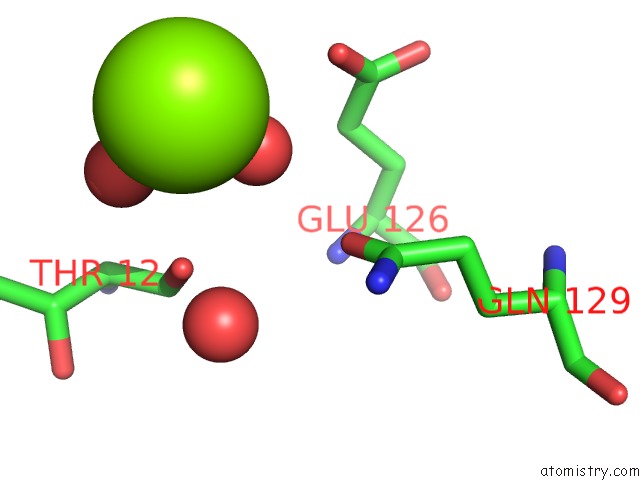
Mono view
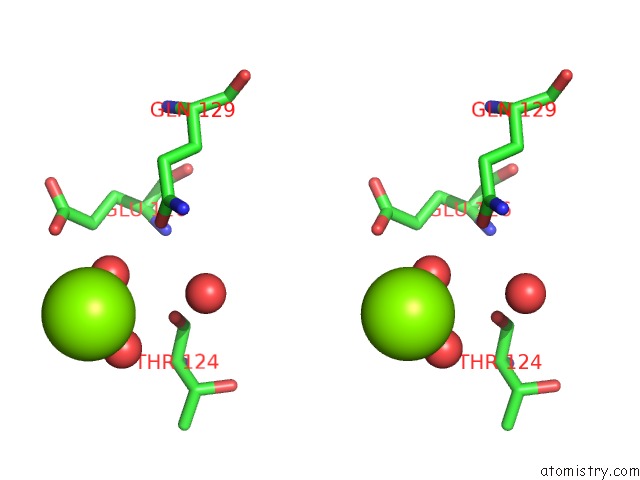
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of H-Ras Q61L in Complex with Gppnhp (State 1) After Structural Transition By Humidity Control within 5.0Å range:
|
Reference:
S.Matsumoto,
H.Taniguchi-Tamura,
M.Araki,
T.Kawamura,
R.Miyamoto,
C.Tsuda,
F.Shima,
T.Kumasaka,
Y.Okuno,
T.Kataoka.
Oncogenic Mutations Q61L and Q61H Confer Active Form-Like Structural Features to the Inactive State (State 1) Conformation of H-Ras Protein. Biochem.Biophys.Res.Commun. V. 565 85 2021.
ISSN: ESSN 1090-2104
PubMed: 34102474
DOI: 10.1016/J.BBRC.2021.05.084
Page generated: Wed Oct 2 15:58:16 2024
ISSN: ESSN 1090-2104
PubMed: 34102474
DOI: 10.1016/J.BBRC.2021.05.084
Last articles
Fe in 2B3YFe in 2B24
Fe in 2B1X
Fe in 2B65
Fe in 2B5H
Fe in 2B4Z
Fe in 2B3X
Fe in 2B11
Fe in 2B12
Fe in 2B10