Magnesium »
PDB 7p8v-7plh »
7pax »
Magnesium in PDB 7pax: Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp
Enzymatic activity of Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp
All present enzymatic activity of Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp:
2.5.1.87;
2.5.1.87;
Protein crystallography data
The structure of Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp, PDB code: 7pax
was solved by
M.Giladi,
M.Lisnyansky Bar-El,
Y.Haitin,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.13 / 2.00 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 183.987, 183.987, 112.537, 90, 90, 120 |
| R / Rfree (%) | 20.5 / 23.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp
(pdb code 7pax). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp, PDB code: 7pax:
In total only one binding site of Magnesium was determined in the Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp, PDB code: 7pax:
Magnesium binding site 1 out of 1 in 7pax
Go back to
Magnesium binding site 1 out
of 1 in the Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp

Mono view
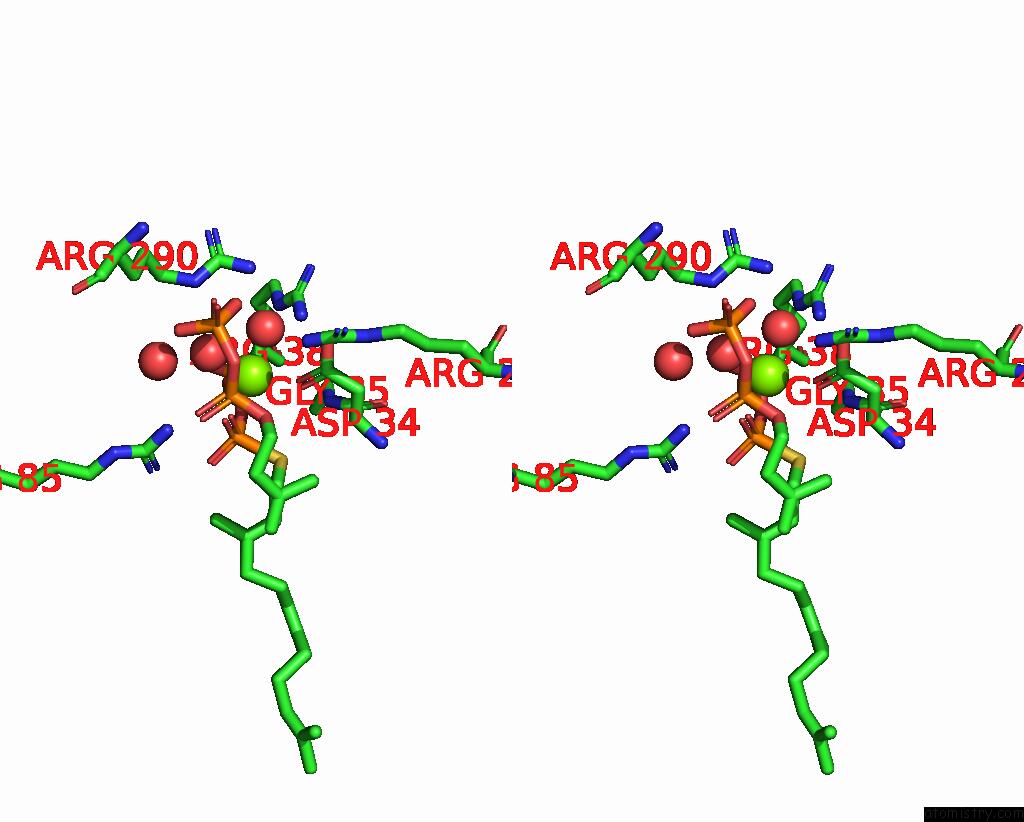
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the Human Heterotetrameric Cis-Prenyltransferase Complex in Complex with Magnesium, Fspp and Ipp within 5.0Å range:
|
Reference:
M.Giladi,
M.Lisnyansky Bar-El,
P.Vankova,
A.Ferofontov,
E.Melvin,
S.Alkaderi,
D.Kavan,
B.Redko,
E.Haimov,
R.Wiener,
P.Man,
Y.Haitin.
Structural Basis For Long-Chain Isoprenoid Synthesis By Cis -Prenyltransferases. Sci Adv V. 8 N1171 2022.
ISSN: ESSN 2375-2548
PubMed: 35584224
DOI: 10.1126/SCIADV.ABN1171
Page generated: Thu Aug 14 12:46:04 2025
ISSN: ESSN 2375-2548
PubMed: 35584224
DOI: 10.1126/SCIADV.ABN1171
Last articles
Mg in 7S67Mg in 7S65
Mg in 7S66
Mg in 7S61
Mg in 7S60
Mg in 7S5Z
Mg in 7S5Y
Mg in 7S5X
Mg in 7S4X
Mg in 7RY4