Magnesium »
PDB 7re3-7rr4 »
7rla »
Magnesium in PDB 7rla: Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
Enzymatic activity of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
All present enzymatic activity of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.:
3.6.4.6;
3.6.4.6;
Magnesium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Magnesium atom in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. (pdb code 7rla). This binding sites where shown within 5.0 Angstroms radius around Magnesium atom.In total 12 binding sites of Magnesium where determined in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs., PDB code: 7rla:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
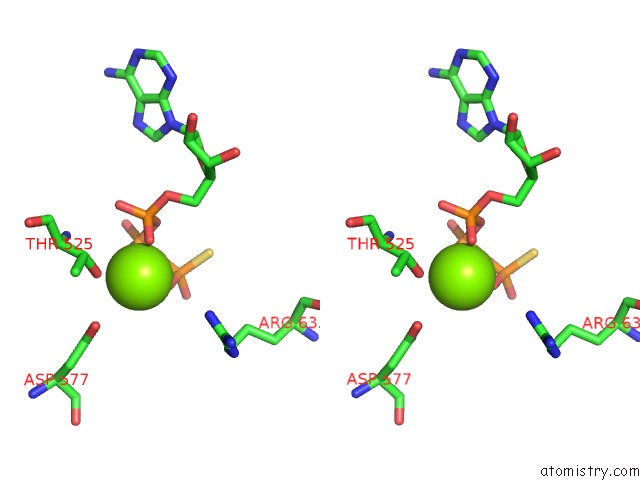
Magnesium binding site 1 out of 12 in 7rla
Go back to
Magnesium binding site 1 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
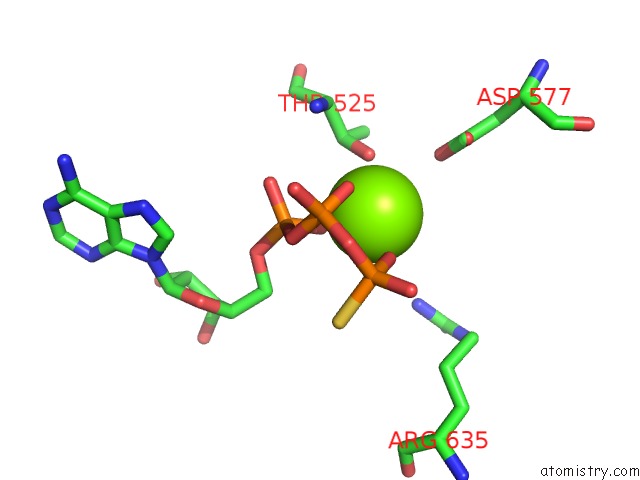
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
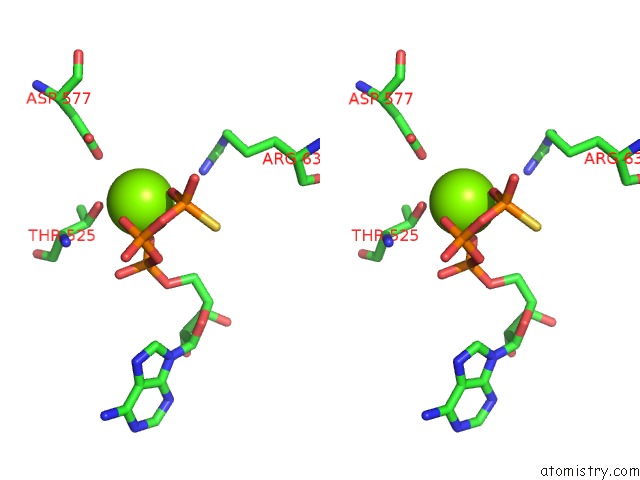
Magnesium binding site 2 out of 12 in 7rla
Go back to
Magnesium binding site 2 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
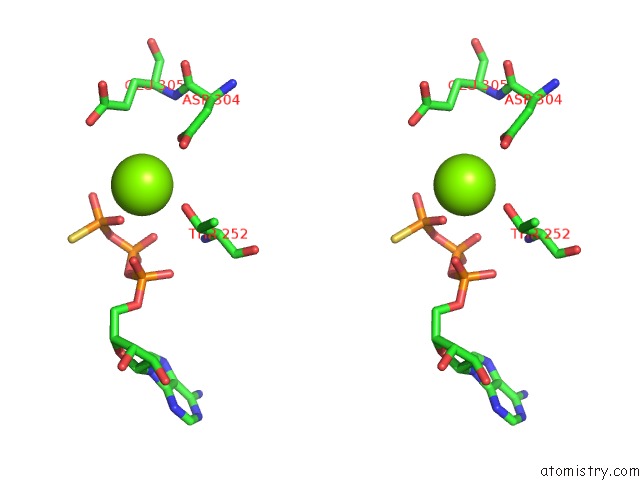
Magnesium binding site 3 out of 12 in 7rla
Go back to
Magnesium binding site 3 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
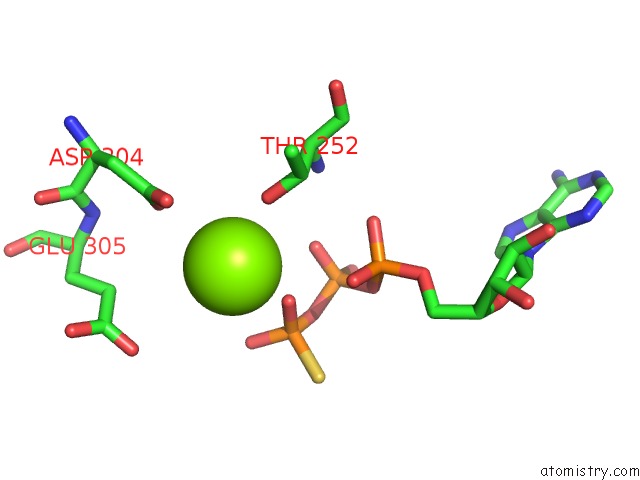
Magnesium binding site 4 out of 12 in 7rla
Go back to
Magnesium binding site 4 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
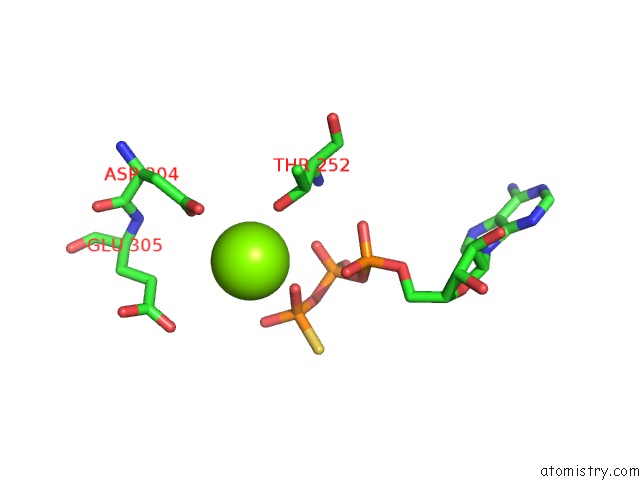
Magnesium binding site 5 out of 12 in 7rla
Go back to
Magnesium binding site 5 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
Magnesium binding site 6 out of 12 in 7rla
Go back to
Magnesium binding site 6 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
Magnesium binding site 7 out of 12 in 7rla
Go back to
Magnesium binding site 7 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
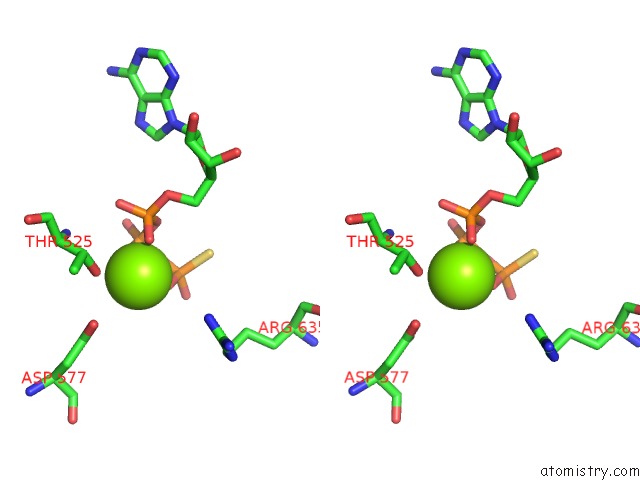
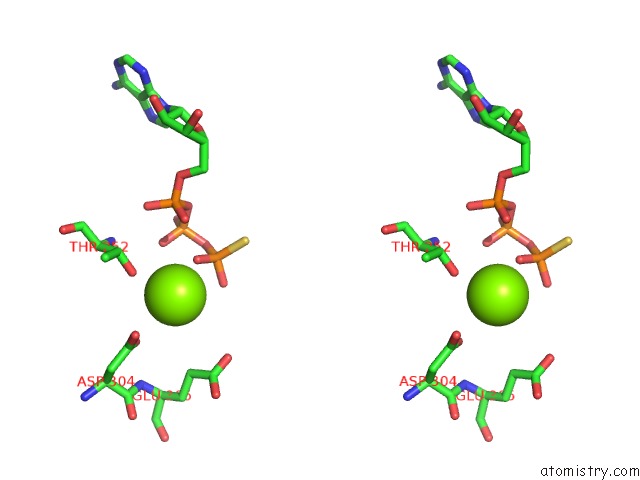
Magnesium binding site 8 out of 12 in 7rla
Go back to
Magnesium binding site 8 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
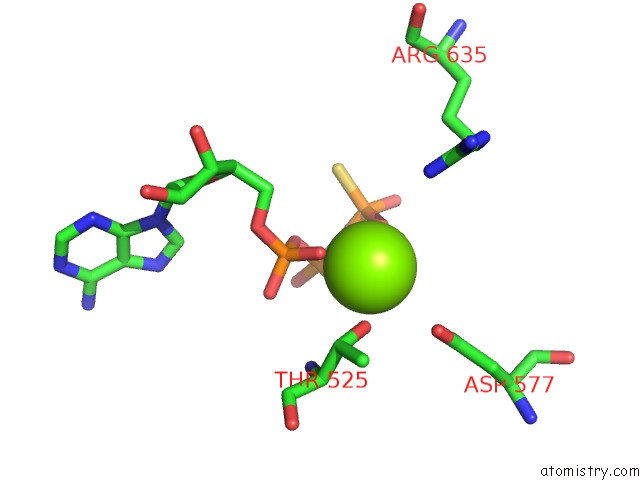
Magnesium binding site 9 out of 12 in 7rla
Go back to
Magnesium binding site 9 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
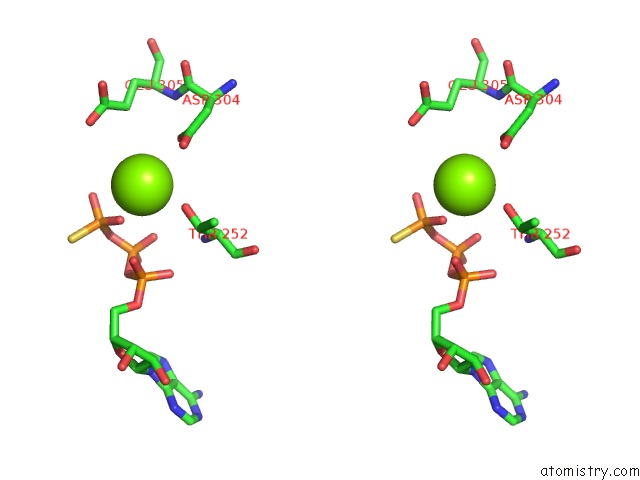
Magnesium binding site 10 out of 12 in 7rla
Go back to
Magnesium binding site 10 out
of 12 in the Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs.

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 10 of Cryo-Em Structure of Human P97-R191Q Mutant Bound to Atpgs. within 5.0Å range:
|
Reference:
B.Caffrey,
X.Zhu,
A.Berezuk,
K.Tuttle,
S.Chittori,
S.Subramaniam.
Common Mutations of Aaa Atpase P97 and Inhibitor Binding Disrupt Inter-Domain Coupling and Subsequent Allosteric Activation J.Biol.Chem. 2021.
ISSN: ESSN 1083-351X
DOI: 10.1016/J.JBC.2021.101187
Page generated: Thu Oct 3 07:58:58 2024
ISSN: ESSN 1083-351X
DOI: 10.1016/J.JBC.2021.101187
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1