Magnesium »
PDB 7syv-7te5 »
7tbv »
Magnesium in PDB 7tbv: Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans
Protein crystallography data
The structure of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans, PDB code: 7tbv
was solved by
P.J.Stogios,
E.Evdokimova,
K.Michalska,
R.Di Leo,
A.Savchenko,
A.Joachimiak,
K.J.F.Satchell,
Center For Structural Genomics Ofinfectious Diseases (Csgid),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.82 / 2.30 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.682, 89.239, 270.708, 90, 90.28, 90 |
| R / Rfree (%) | 17.8 / 22.6 |
Other elements in 7tbv:
The structure of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans
(pdb code 7tbv). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans, PDB code: 7tbv:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans, PDB code: 7tbv:
Jump to Magnesium binding site number: 1; 2; 3; 4;
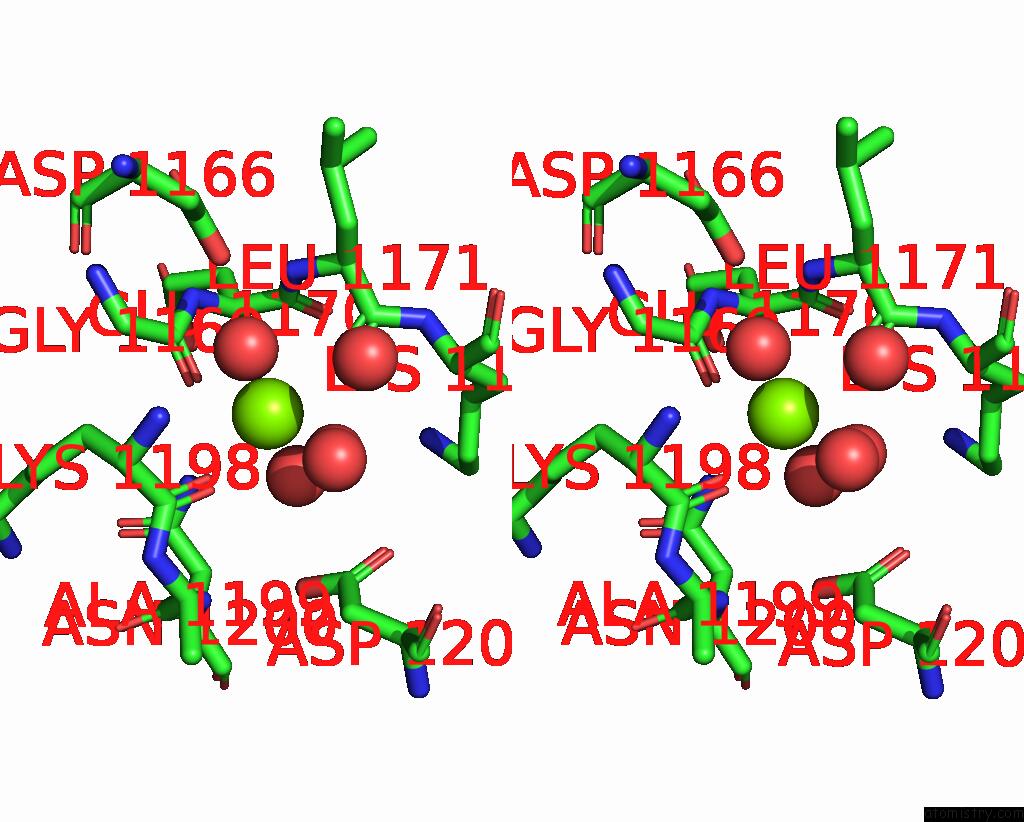
Magnesium binding site 1 out of 4 in 7tbv
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans within 5.0Å range:
|
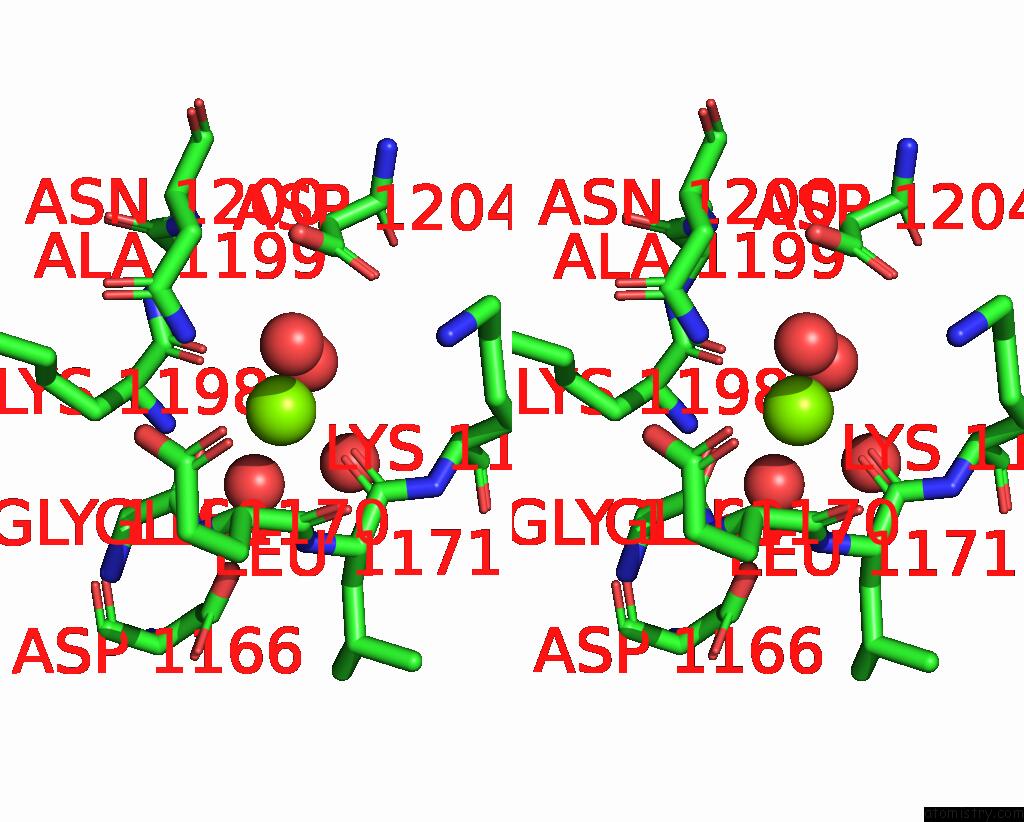
Magnesium binding site 2 out of 4 in 7tbv
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans within 5.0Å range:
|
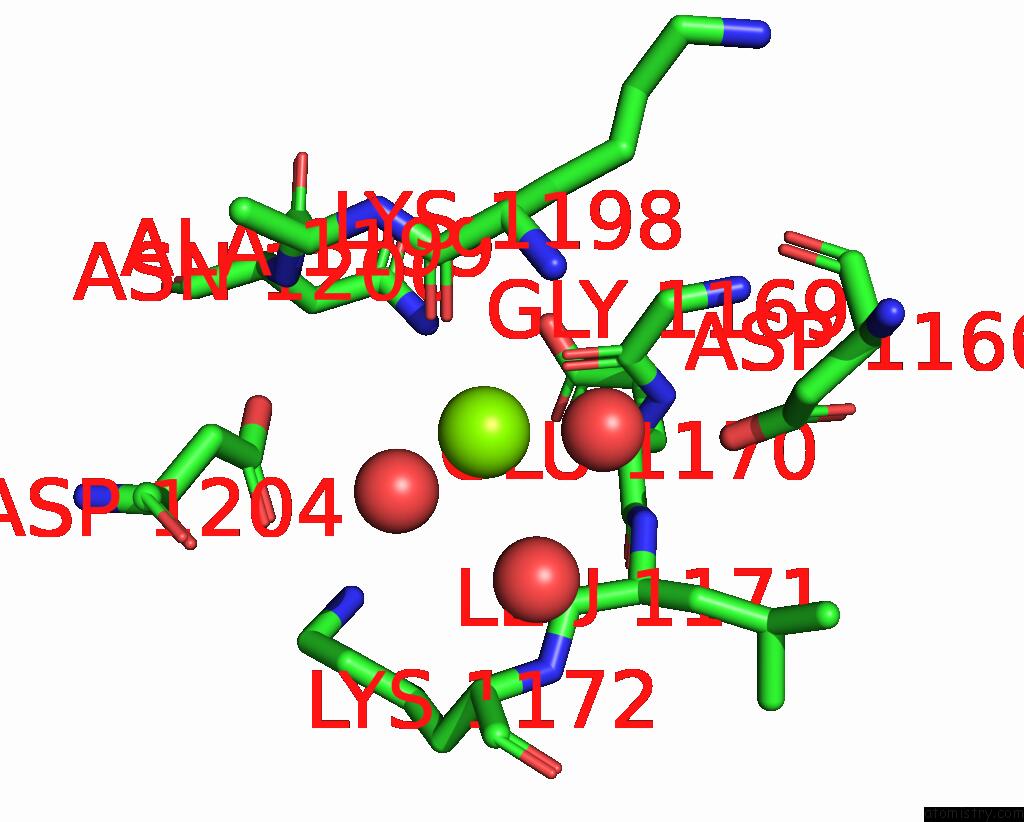
Magnesium binding site 3 out of 4 in 7tbv
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans within 5.0Å range:
|
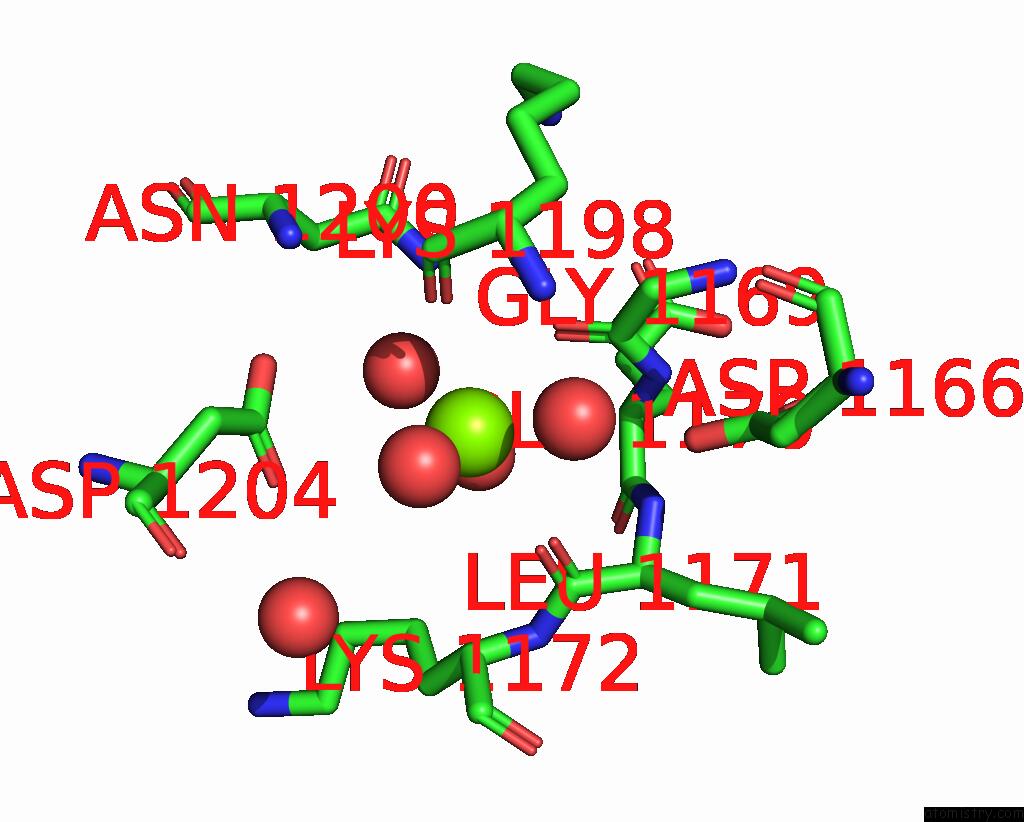
Magnesium binding site 4 out of 4 in 7tbv
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of the Shikimate Kinase + 3-Dehydroquinate Dehydratase + 3-Dehydroshikimate Dehydrogenase Domains of ARO1 From Candida Albicans within 5.0Å range:
|
Reference:
P.J.Stogios,
S.D.Liston,
C.Semper,
B.Quade,
K.Michalska,
E.Evdokimova,
S.Ram,
Z.Otwinowski,
D.Borek,
L.E.Cowen,
A.Savchenko.
Molecular Analysis and Essentiality of ARO1 Shikimate Biosynthesis Multi-Enzyme in Candida Albicans. Life Sci Alliance V. 5 2022.
ISSN: ESSN 2575-1077
PubMed: 35512834
DOI: 10.26508/LSA.202101358
Page generated: Thu Oct 3 09:04:58 2024
ISSN: ESSN 2575-1077
PubMed: 35512834
DOI: 10.26508/LSA.202101358
Last articles
Cl in 6C4JCl in 6C7D
Cl in 6C6Q
Cl in 6C5U
Cl in 6C73
Cl in 6C58
Cl in 6C5I
Cl in 6C4D
Cl in 6C5H
Cl in 6C5C