Magnesium »
PDB 7tr7-7u0y »
7tu6 »
Magnesium in PDB 7tu6: Structure of the L. Blandensis Dgtpase Bound to Datp
Enzymatic activity of Structure of the L. Blandensis Dgtpase Bound to Datp
All present enzymatic activity of Structure of the L. Blandensis Dgtpase Bound to Datp:
3.1.5.1;
3.1.5.1;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the L. Blandensis Dgtpase Bound to Datp
(pdb code 7tu6). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Structure of the L. Blandensis Dgtpase Bound to Datp, PDB code: 7tu6:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Structure of the L. Blandensis Dgtpase Bound to Datp, PDB code: 7tu6:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 7tu6
Go back to
Magnesium binding site 1 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp
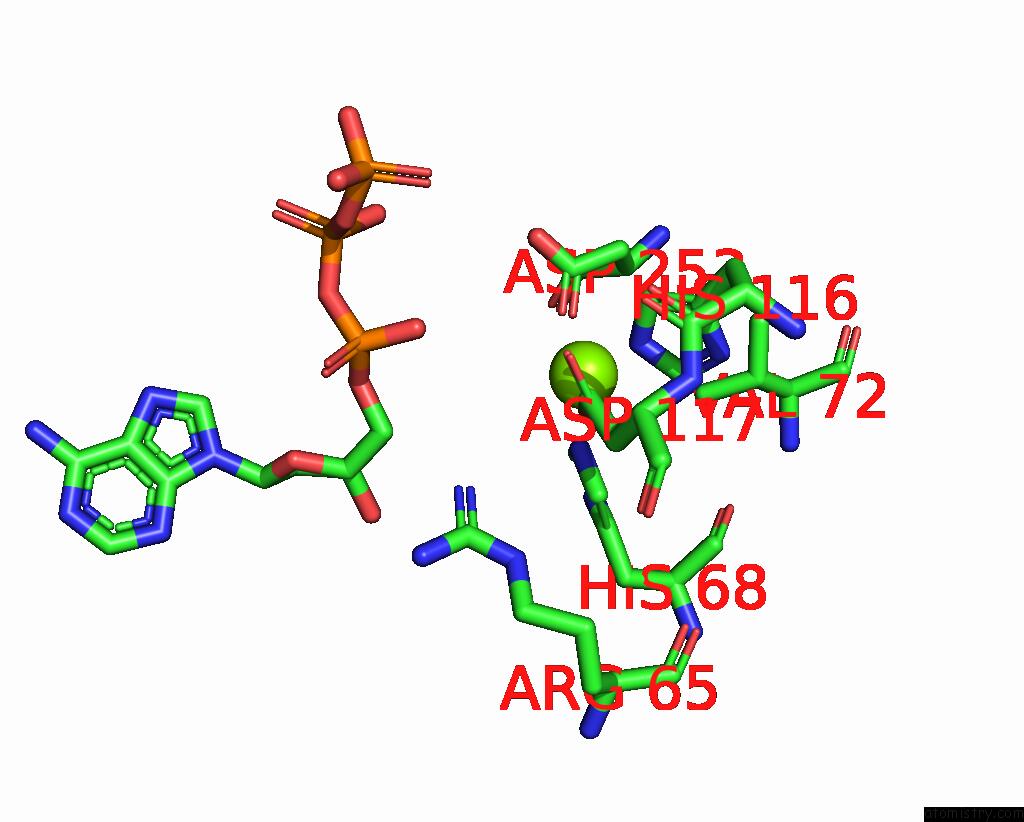
Mono view
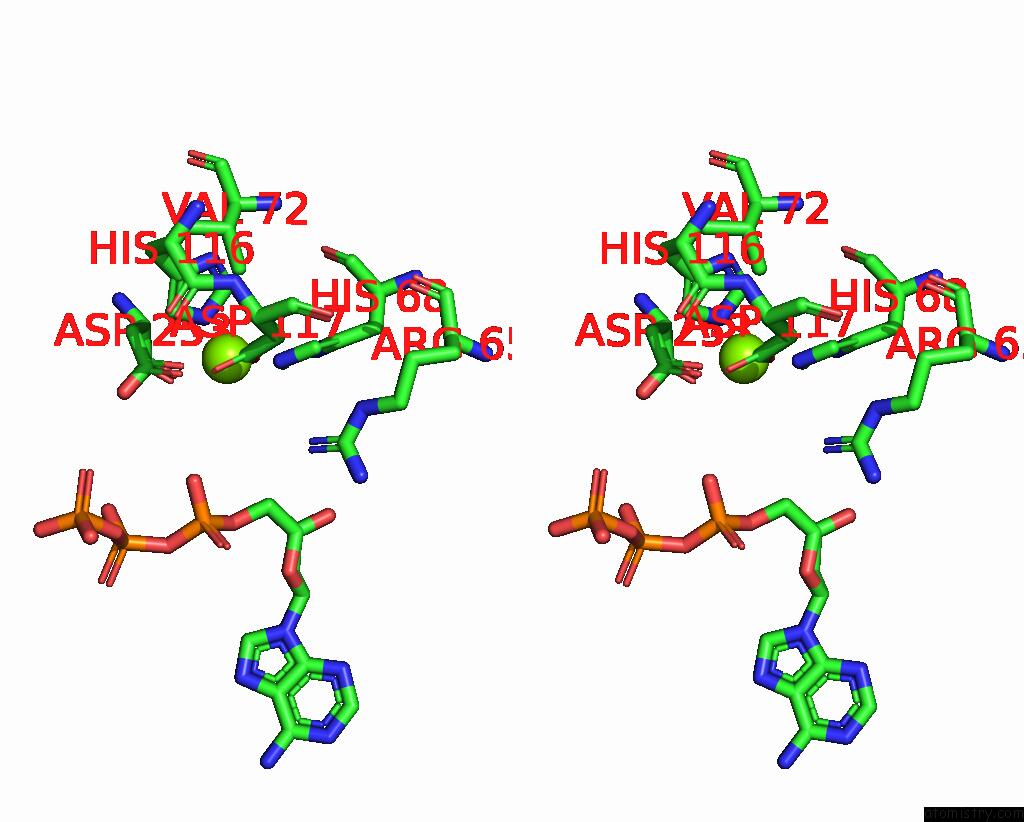
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 7tu6
Go back to
Magnesium binding site 2 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp
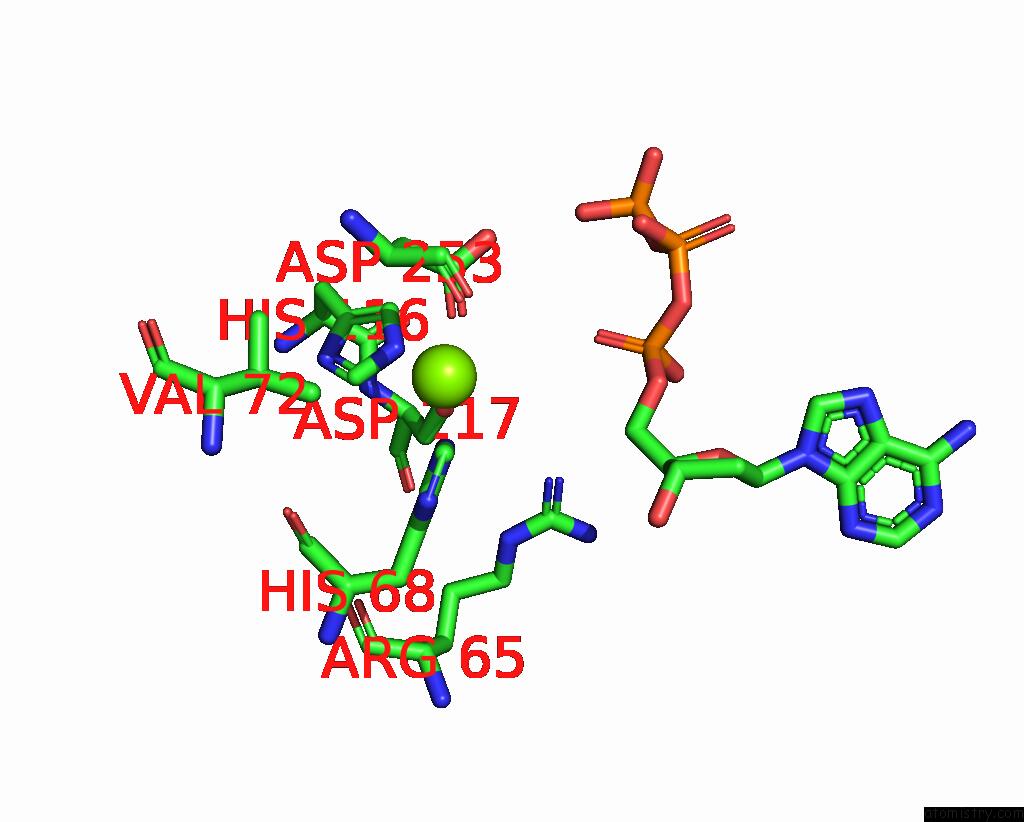
Mono view
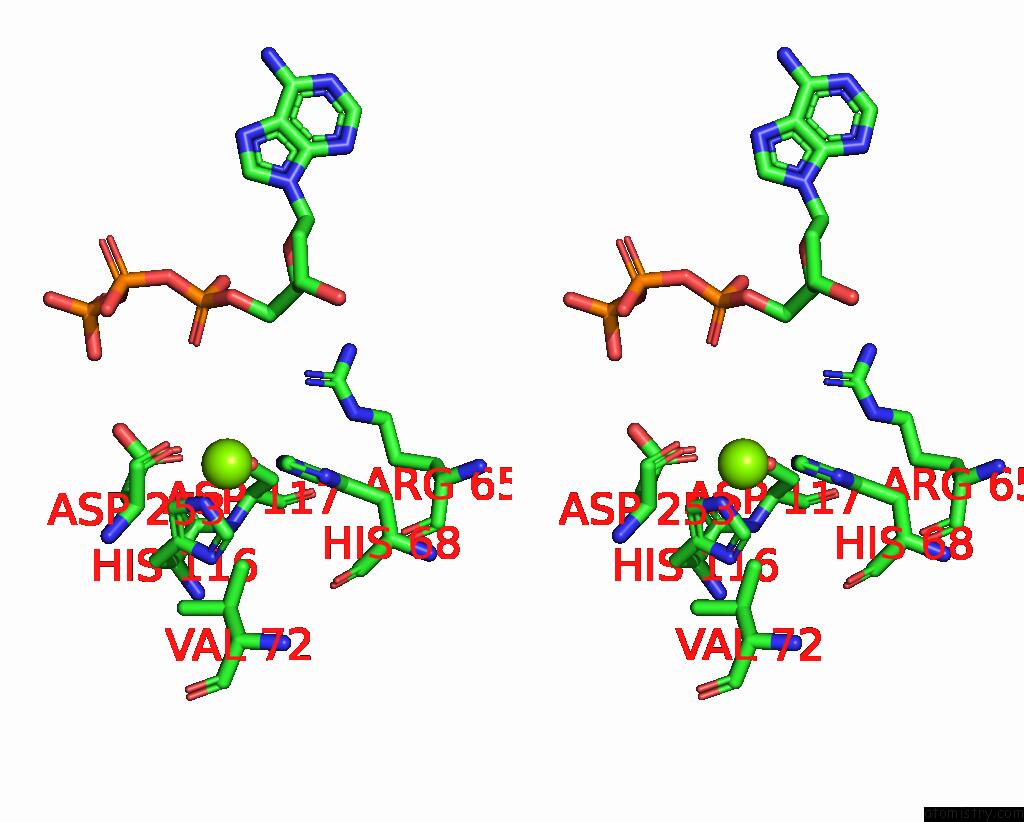
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
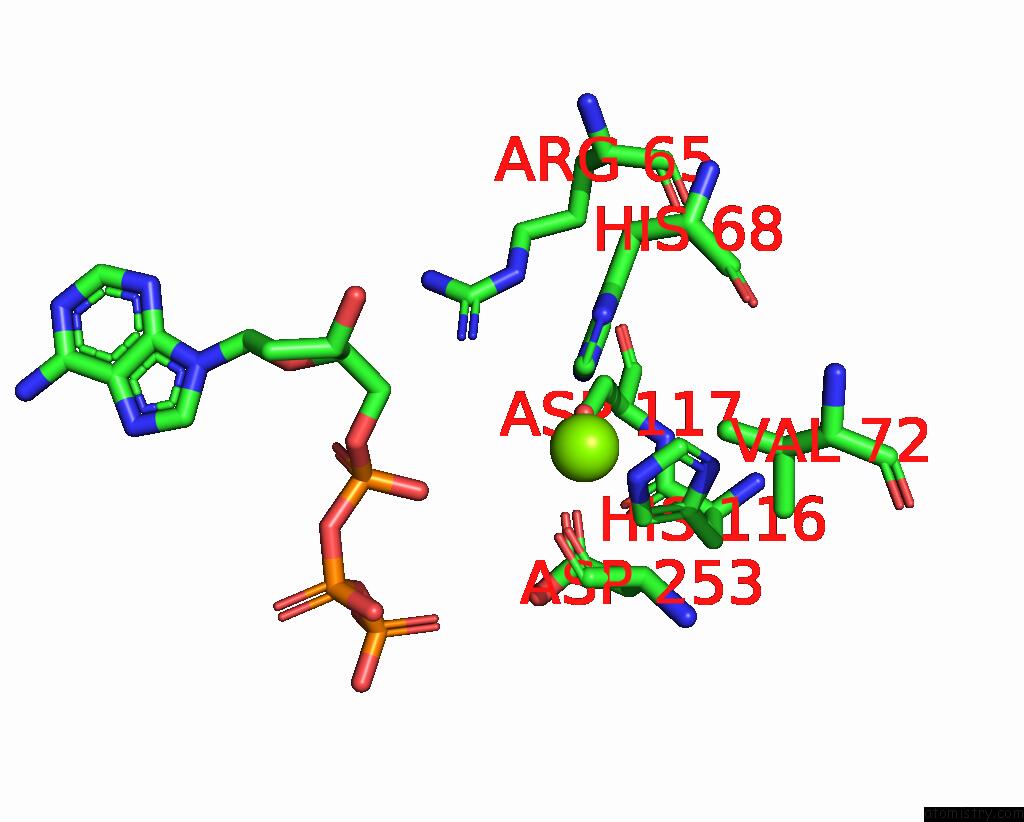
Magnesium binding site 3 out of 6 in 7tu6
Go back to
Magnesium binding site 3 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
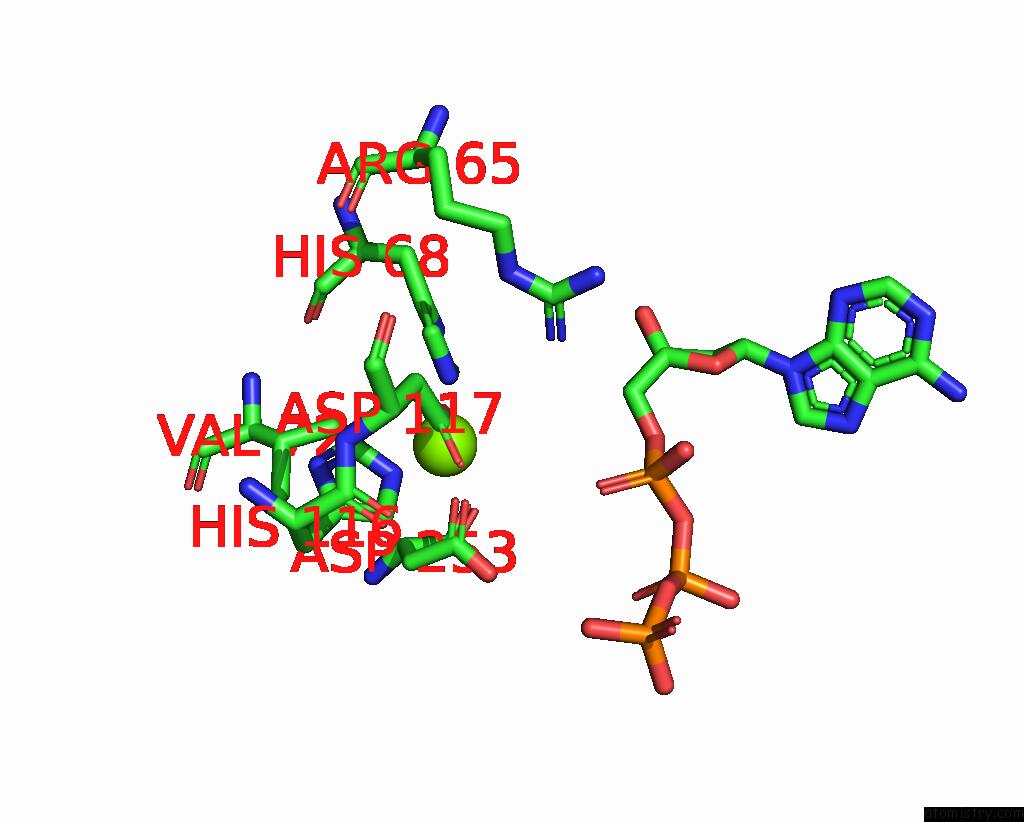
Magnesium binding site 4 out of 6 in 7tu6
Go back to
Magnesium binding site 4 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
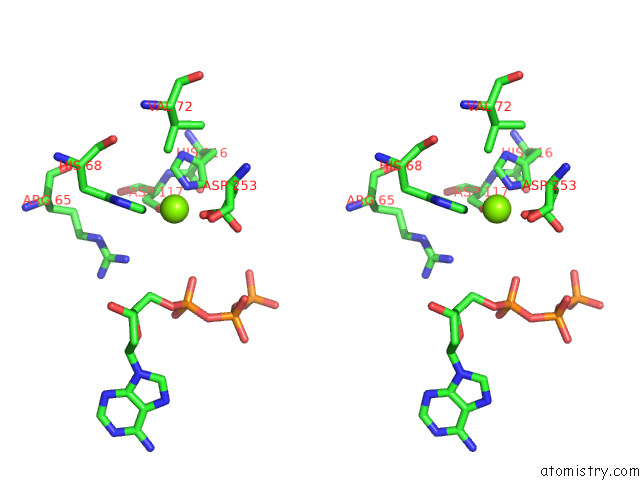
Magnesium binding site 5 out of 6 in 7tu6
Go back to
Magnesium binding site 5 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp

Mono view
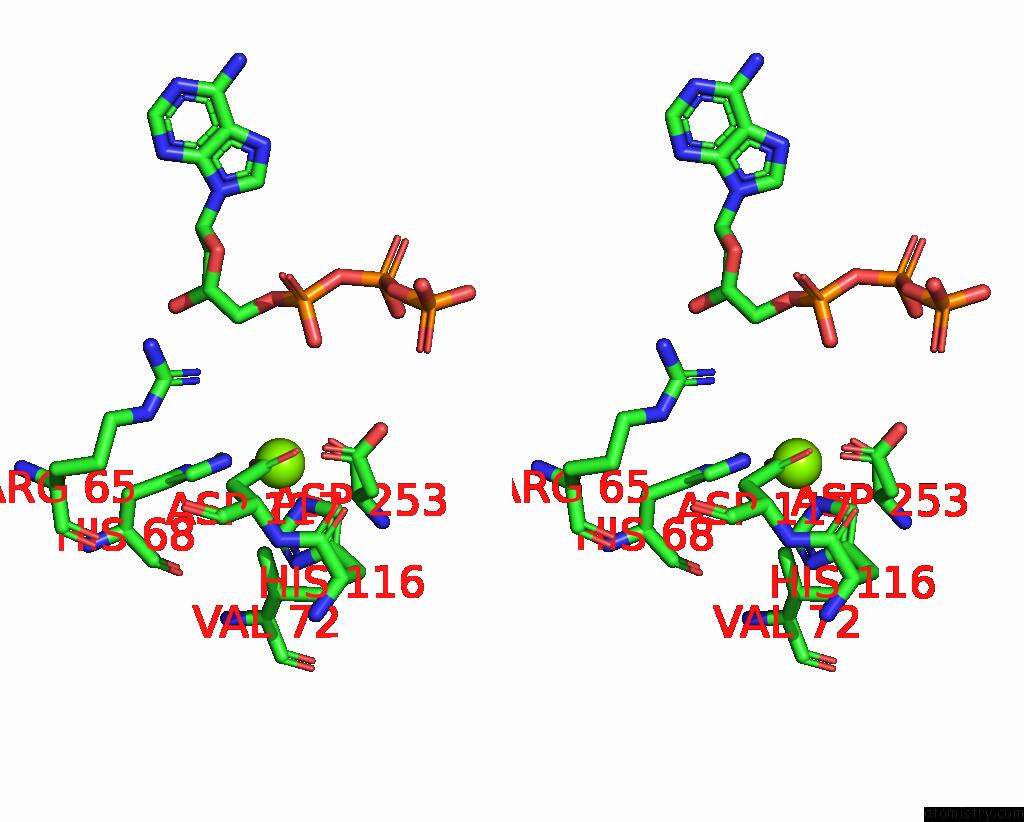
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 7tu6
Go back to
Magnesium binding site 6 out
of 6 in the Structure of the L. Blandensis Dgtpase Bound to Datp
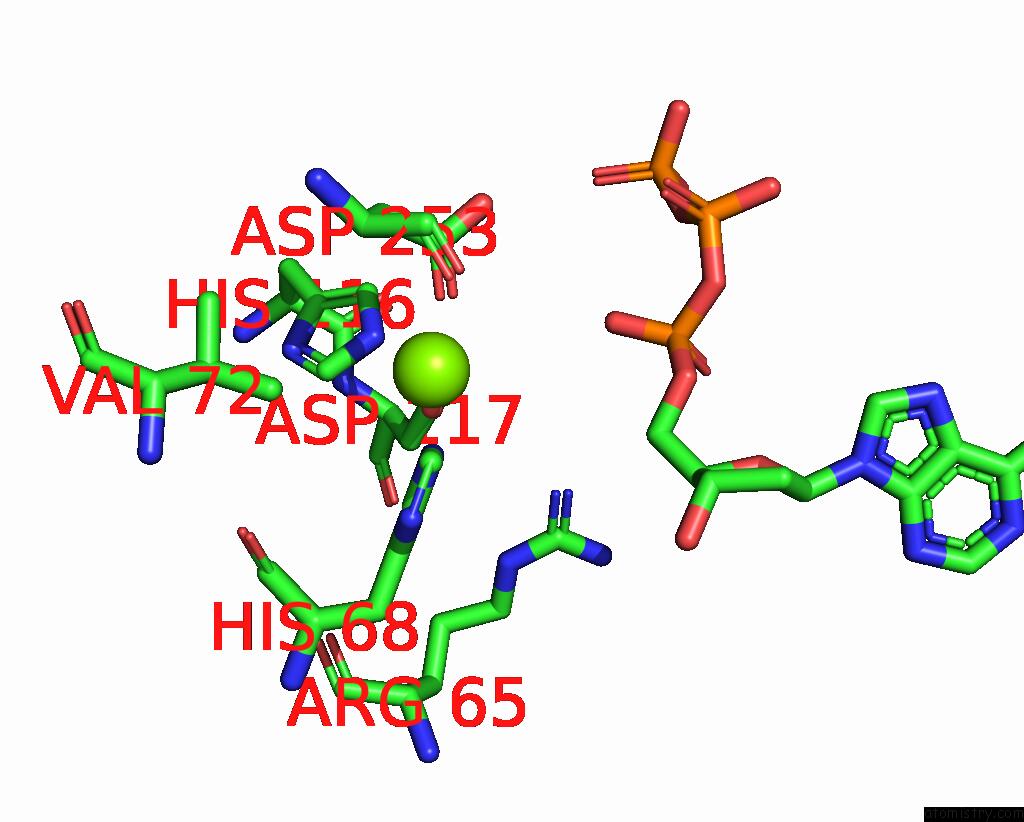
Mono view
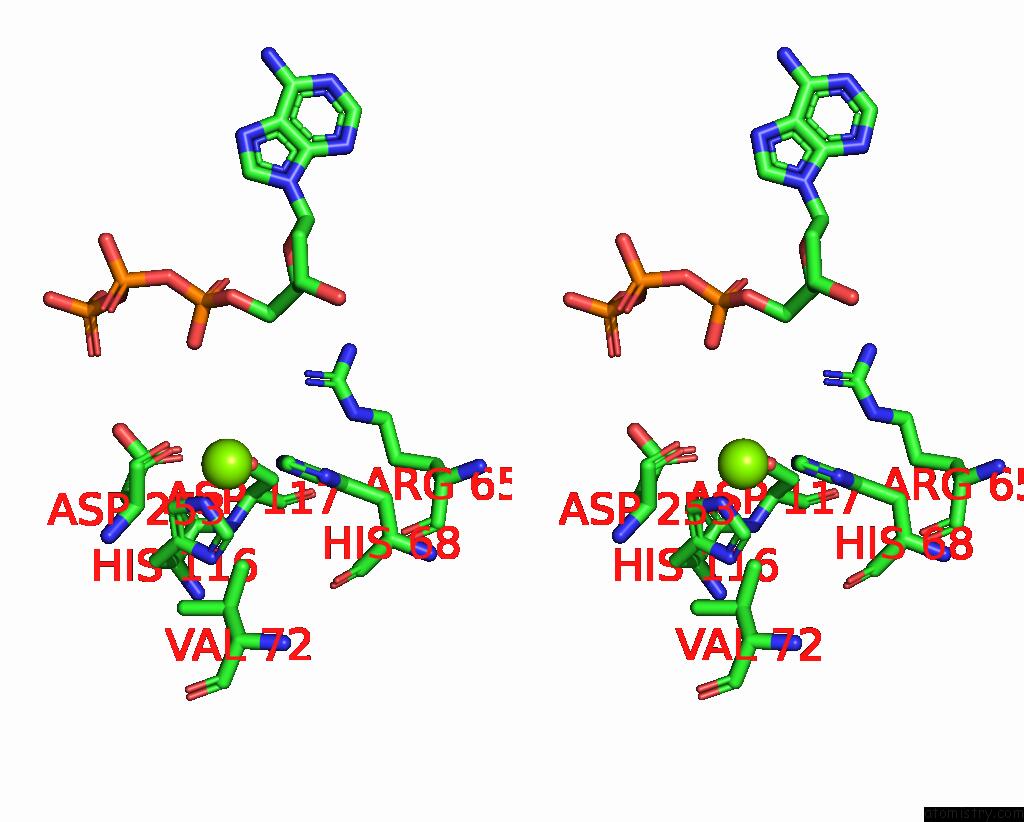
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structure of the L. Blandensis Dgtpase Bound to Datp within 5.0Å range:
|
Reference:
B.P.Klemm,
A.P.Sikkema,
A.L.Hsu,
J.C.Horng,
T.M.T.Hall,
M.J.Borgnia,
R.M.Schaaper.
High-Resolution Structures of the SAMHD1 Dgtpase Homolog From Leeuwenhoekiella Blandensis Reveal A Novel Mechanism of Allosteric Activation By Datp. J.Biol.Chem. V. 298 02073 2022.
ISSN: ESSN 1083-351X
PubMed: 35643313
DOI: 10.1016/J.JBC.2022.102073
Page generated: Thu Oct 3 09:29:36 2024
ISSN: ESSN 1083-351X
PubMed: 35643313
DOI: 10.1016/J.JBC.2022.102073
Last articles
Cl in 7WKRCl in 7WGP
Cl in 7WK1
Cl in 7WHH
Cl in 7WGT
Cl in 7WF6
Cl in 7WGL
Cl in 7WGO
Cl in 7WGD
Cl in 7WFR