Magnesium »
PDB 7tr7-7u0y »
7tu8 »
Magnesium in PDB 7tu8: Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
Enzymatic activity of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
All present enzymatic activity of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp:
3.1.5.1;
3.1.5.1;
Magnesium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Magnesium atom in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp (pdb code 7tu8). This binding sites where shown within 5.0 Angstroms radius around Magnesium atom.In total 12 binding sites of Magnesium where determined in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp, PDB code: 7tu8:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
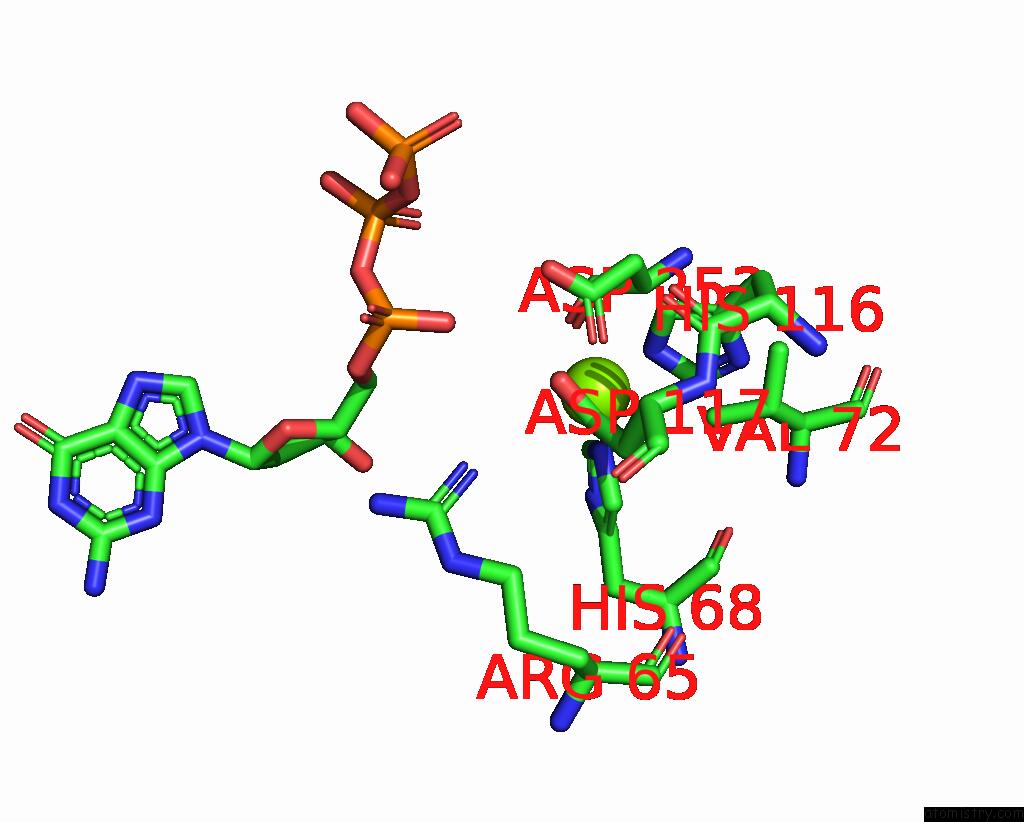
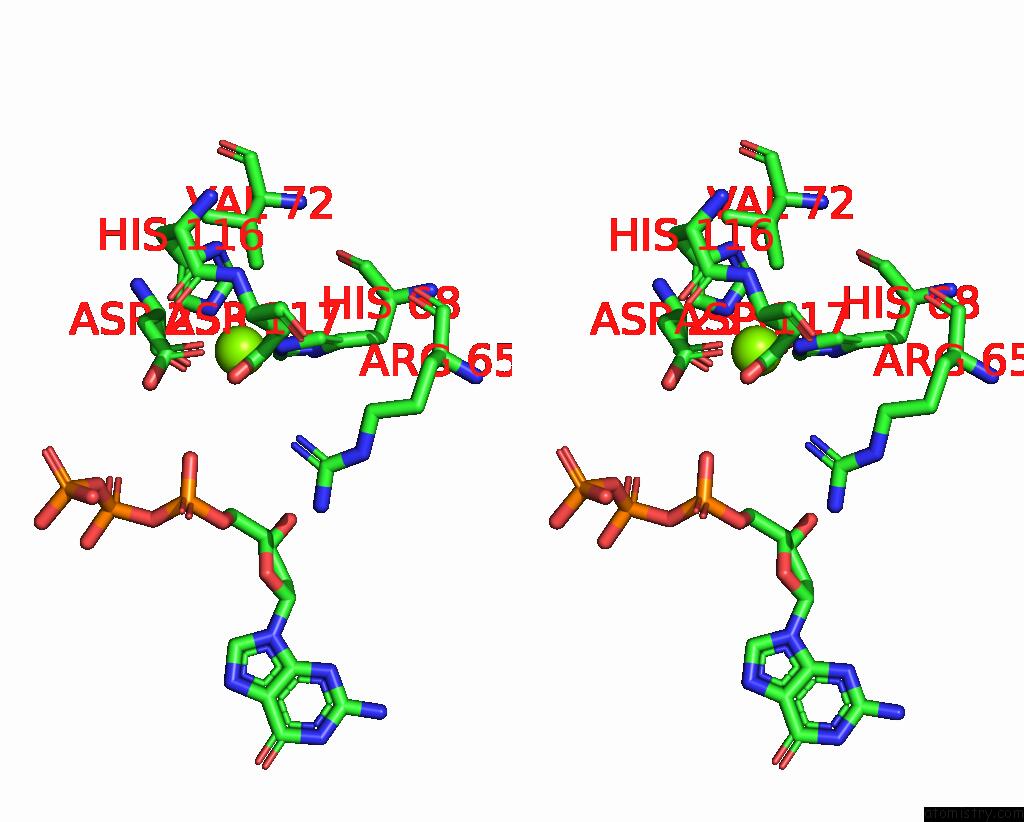
Magnesium binding site 1 out of 12 in 7tu8
Go back to
Magnesium binding site 1 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
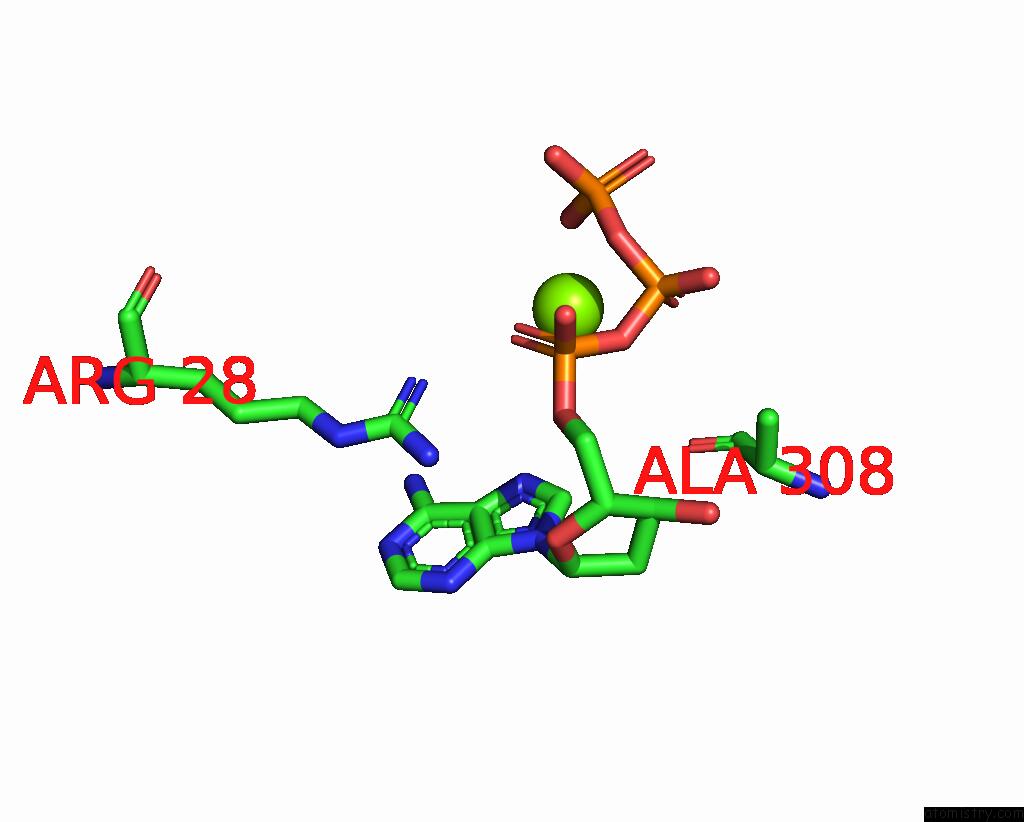
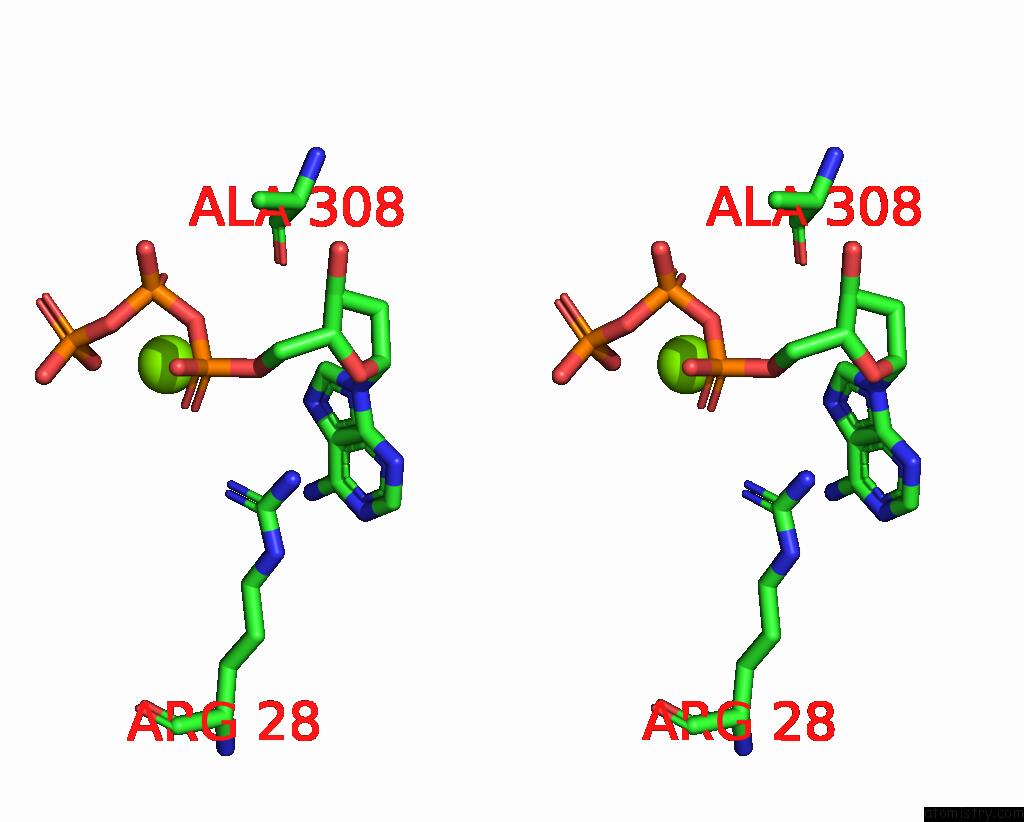
Magnesium binding site 2 out of 12 in 7tu8
Go back to
Magnesium binding site 2 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 3 out of 12 in 7tu8
Go back to
Magnesium binding site 3 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp

Mono view
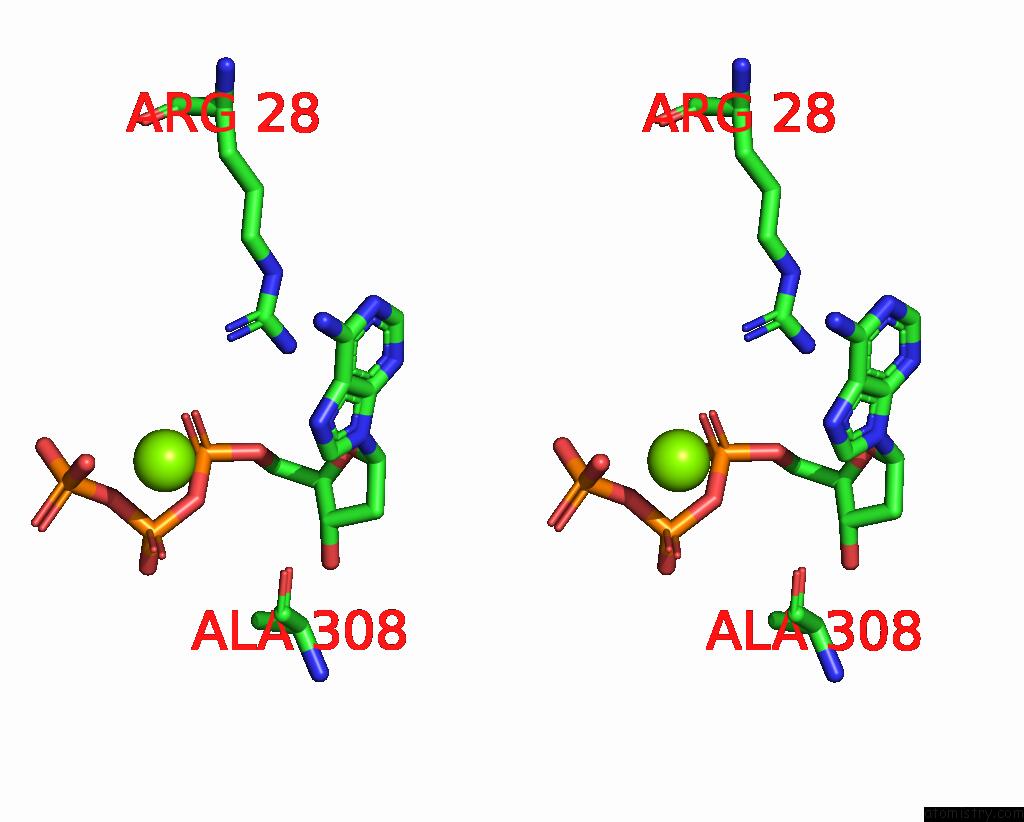
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 4 out of 12 in 7tu8
Go back to
Magnesium binding site 4 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
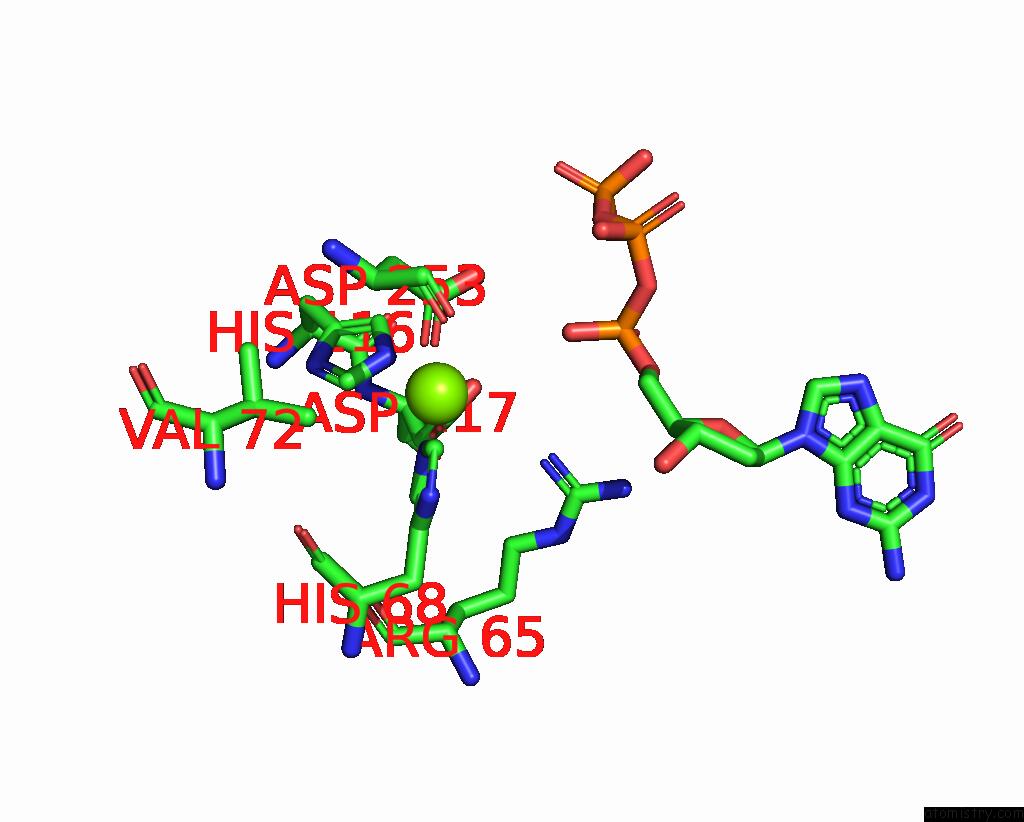
Mono view
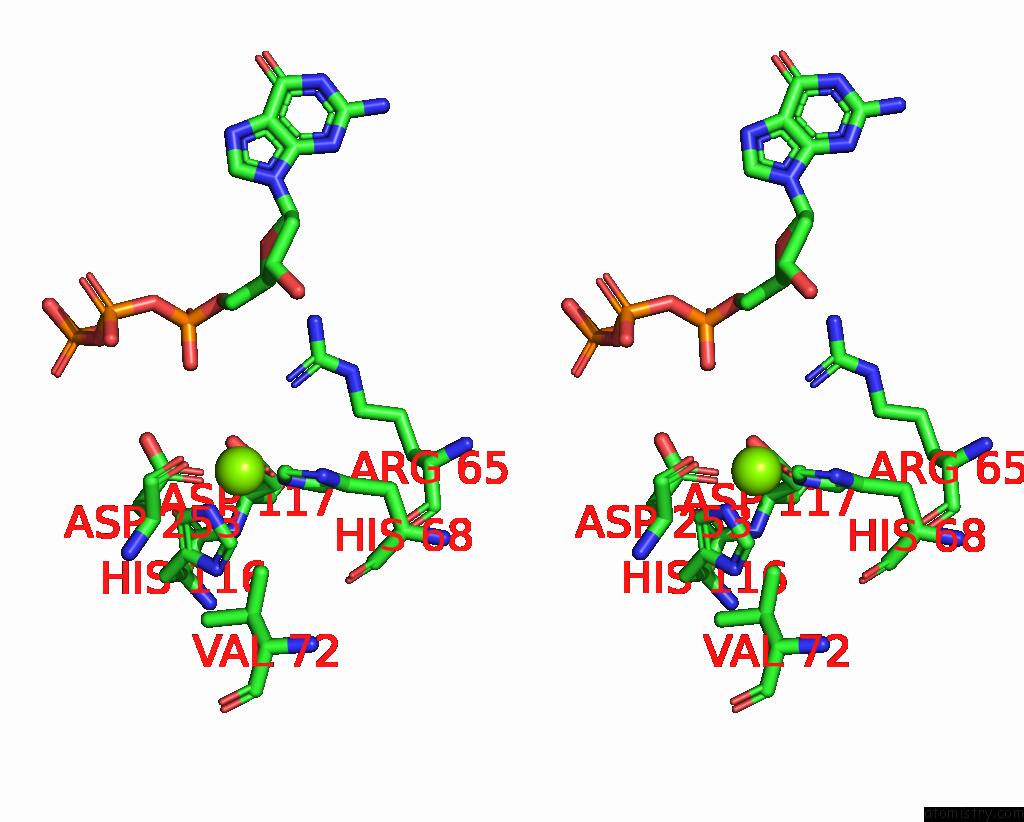
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 5 out of 12 in 7tu8
Go back to
Magnesium binding site 5 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp

Mono view
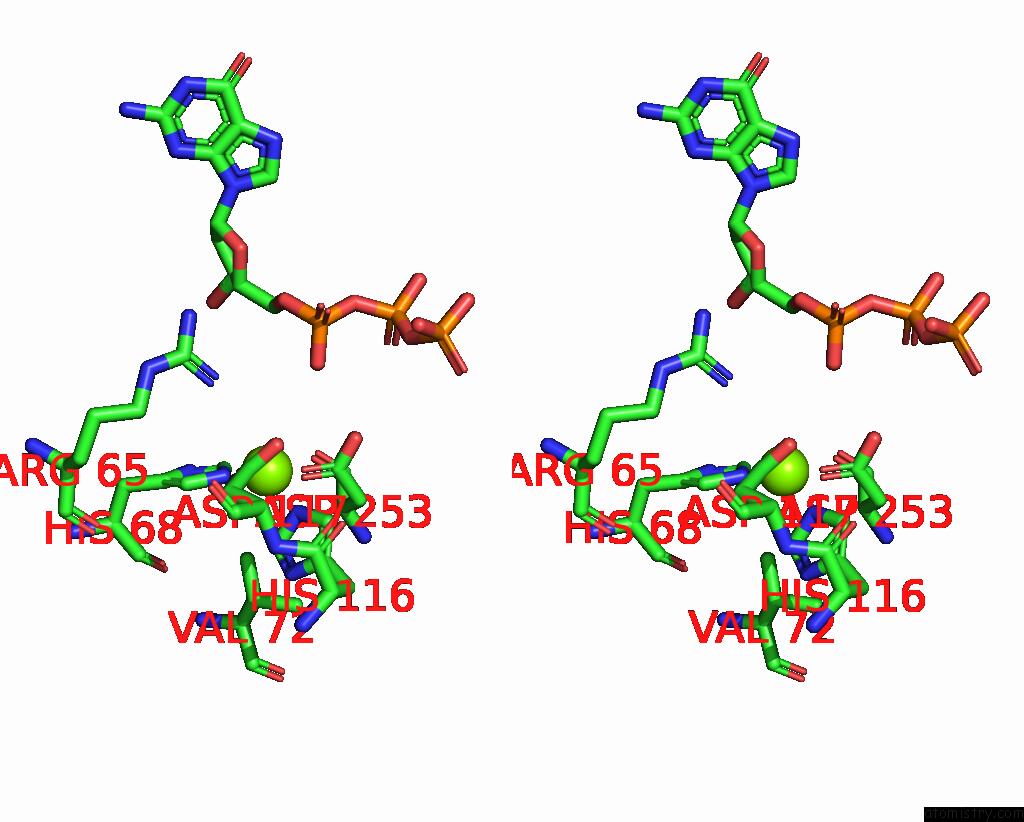
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 6 out of 12 in 7tu8
Go back to
Magnesium binding site 6 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
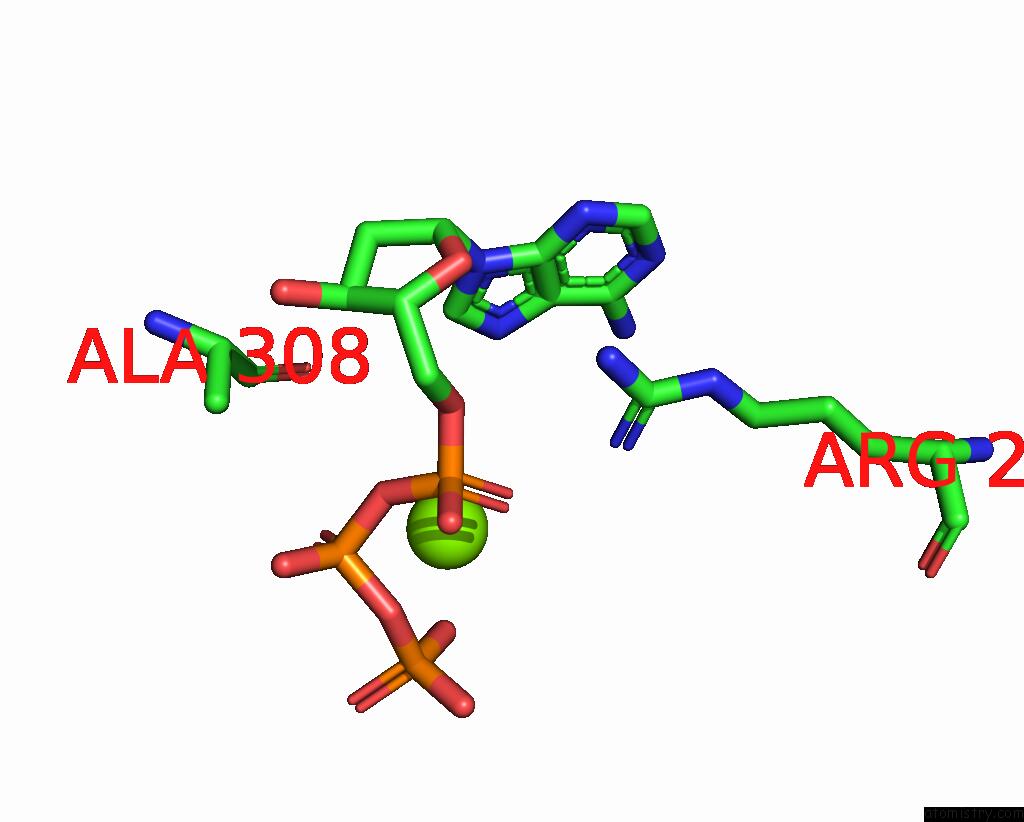
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 7 out of 12 in 7tu8
Go back to
Magnesium binding site 7 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
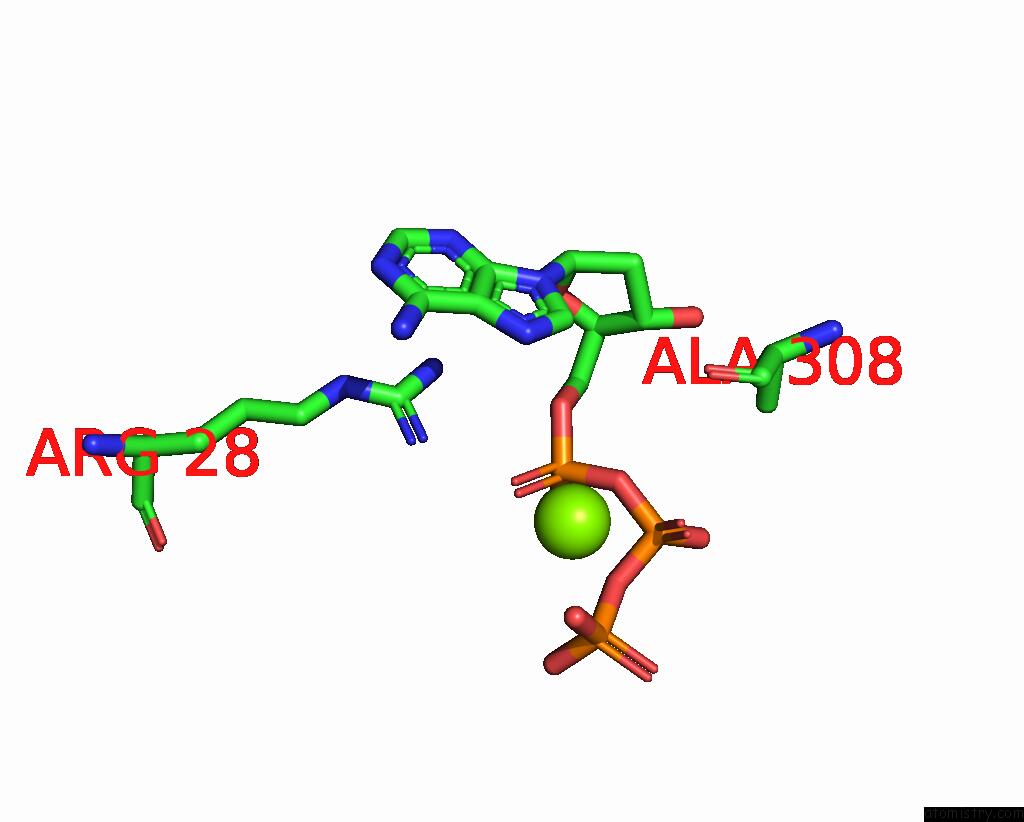
Mono view
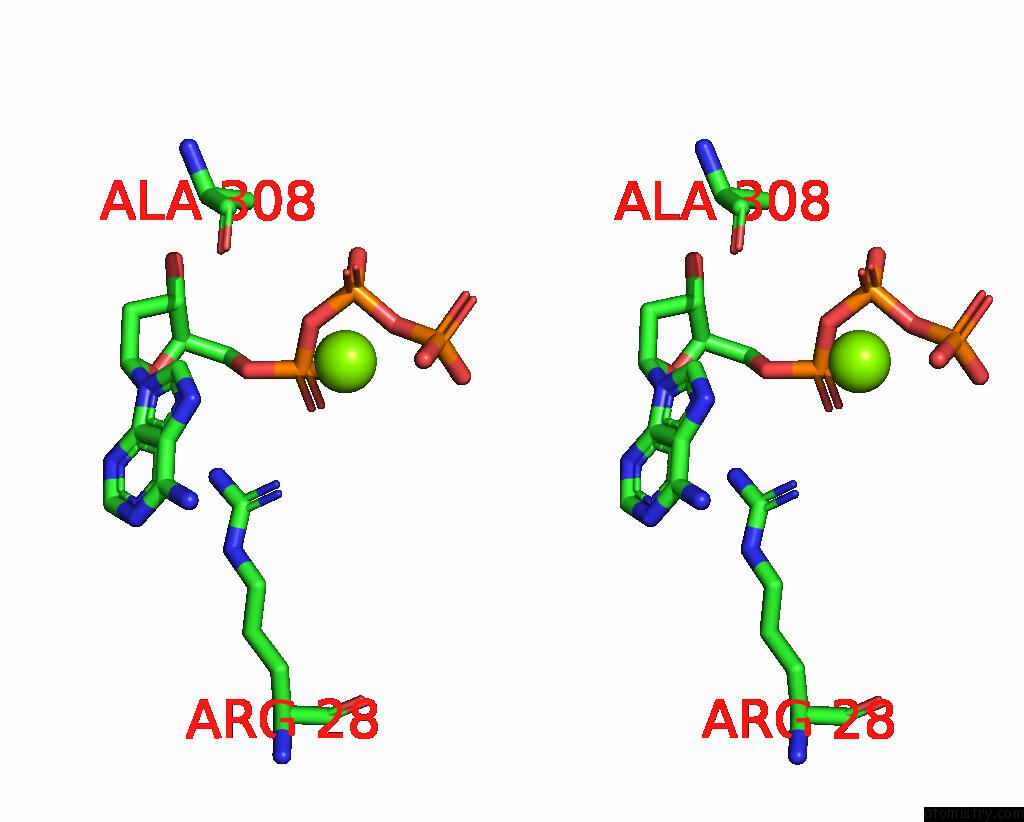
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 8 out of 12 in 7tu8
Go back to
Magnesium binding site 8 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
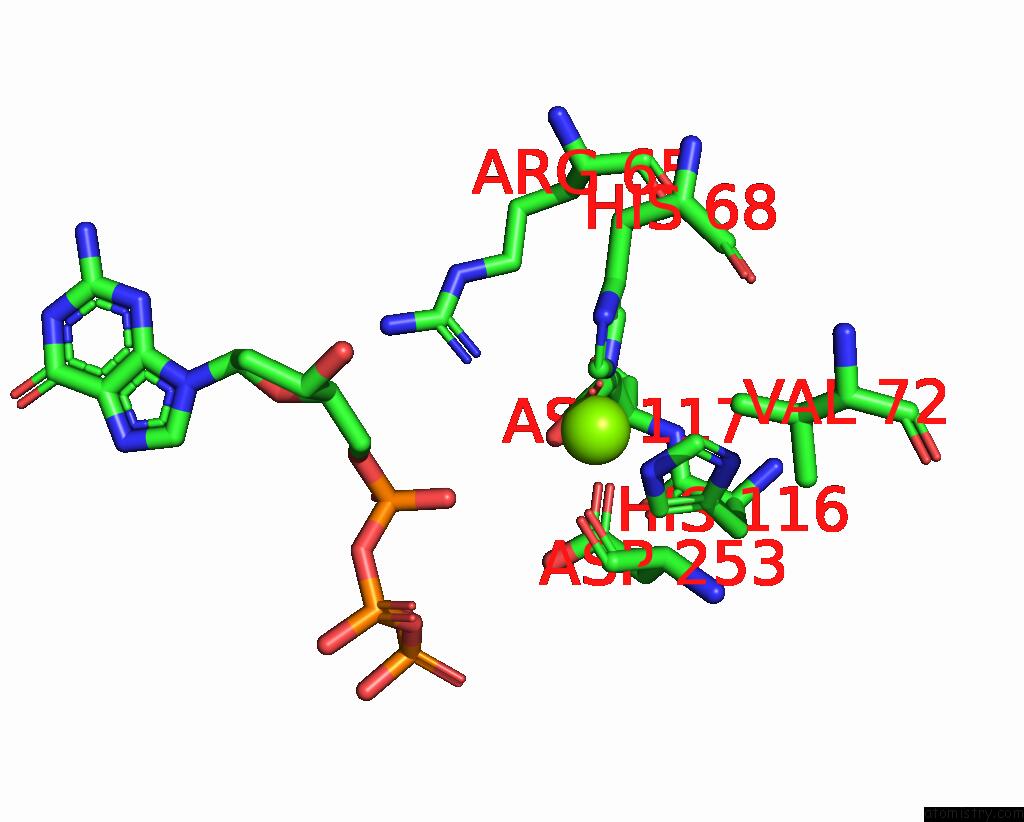
Mono view
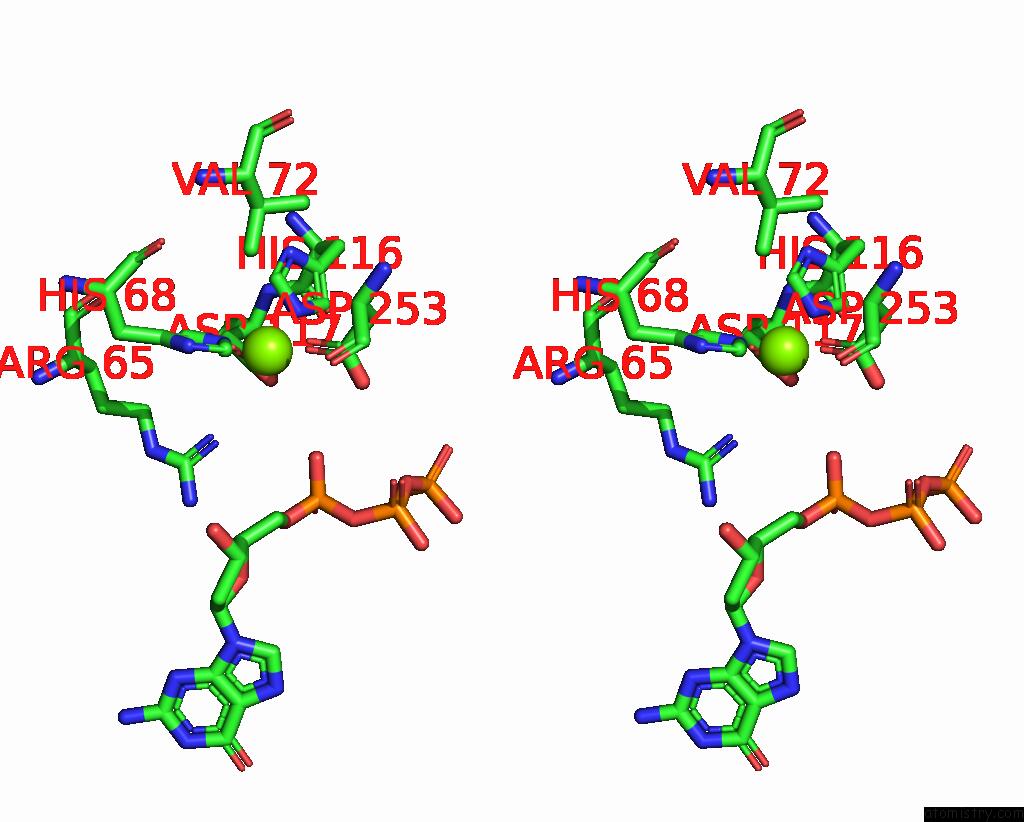
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 9 out of 12 in 7tu8
Go back to
Magnesium binding site 9 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
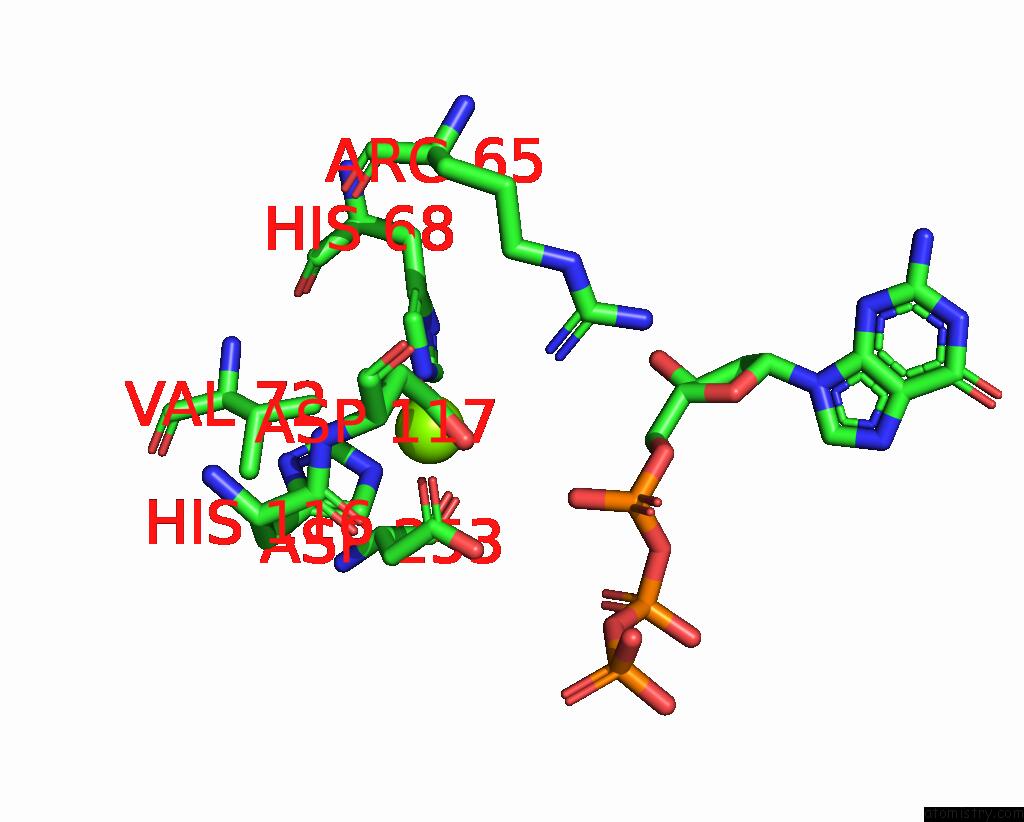
Mono view
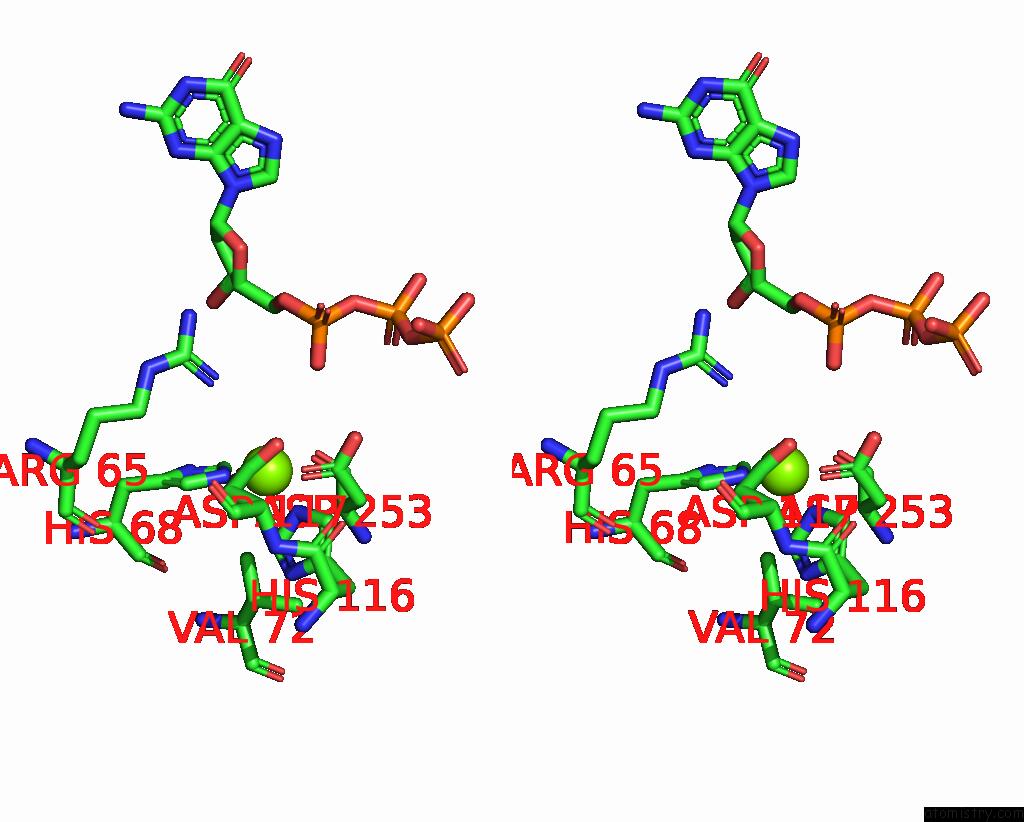
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Magnesium binding site 10 out of 12 in 7tu8
Go back to
Magnesium binding site 10 out
of 12 in the Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp
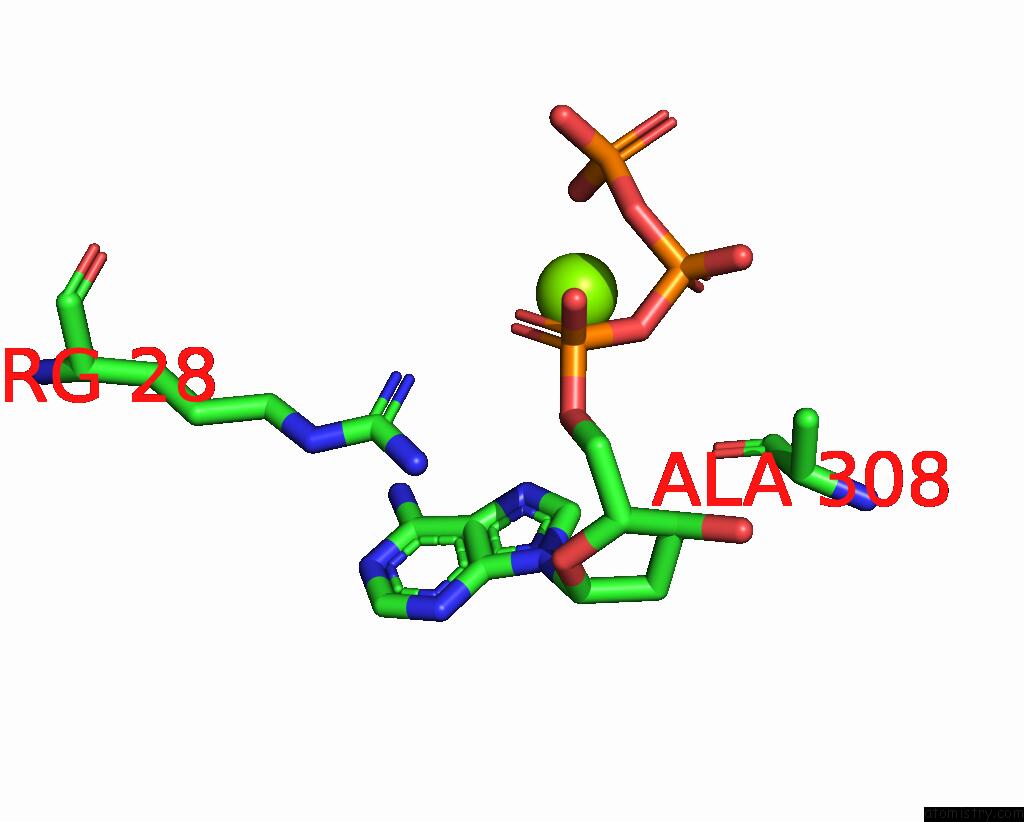
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 10 of Structure of the L. Blandensis Dgtpase H125A Mutant Bound to Dgtp and Datp within 5.0Å range:
|
Reference:
B.P.Klemm,
A.P.Sikkema,
A.L.Hsu,
J.C.Horng,
T.M.T.Hall,
M.J.Borgnia,
R.M.Schaaper.
High-Resolution Structures of the SAMHD1 Dgtpase Homolog From Leeuwenhoekiella Blandensis Reveal A Novel Mechanism of Allosteric Activation By Datp. J.Biol.Chem. V. 298 02073 2022.
ISSN: ESSN 1083-351X
PubMed: 35643313
DOI: 10.1016/J.JBC.2022.102073
Page generated: Thu Oct 3 09:29:36 2024
ISSN: ESSN 1083-351X
PubMed: 35643313
DOI: 10.1016/J.JBC.2022.102073
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO