Magnesium »
PDB 7uo9-7uz0 »
7uta »
Magnesium in PDB 7uta: Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
Enzymatic activity of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
All present enzymatic activity of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction:
1.18.6.1;
1.18.6.1;
Other elements in 7uta:
The structure of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction also contains other interesting chemical elements:
| Molybdenum | (Mo) | 2 atoms |
| Iron | (Fe) | 40 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
(pdb code 7uta). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction, PDB code: 7uta:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction, PDB code: 7uta:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 7uta
Go back to
Magnesium binding site 1 out
of 4 in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
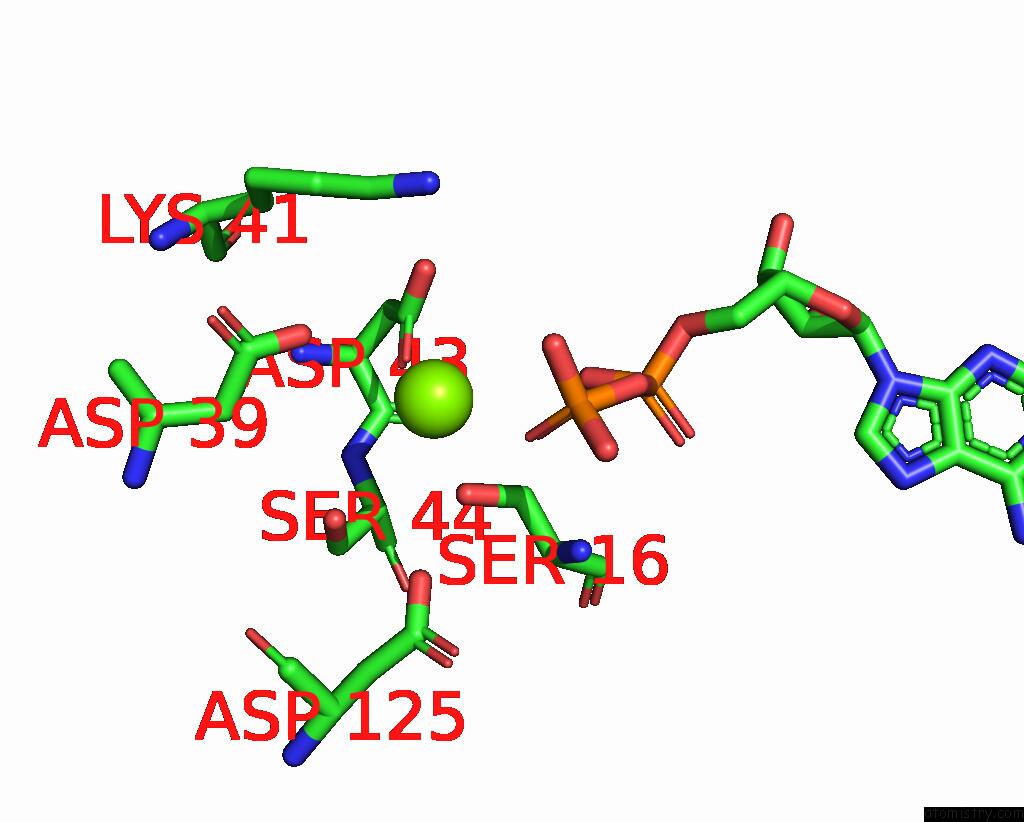
Mono view
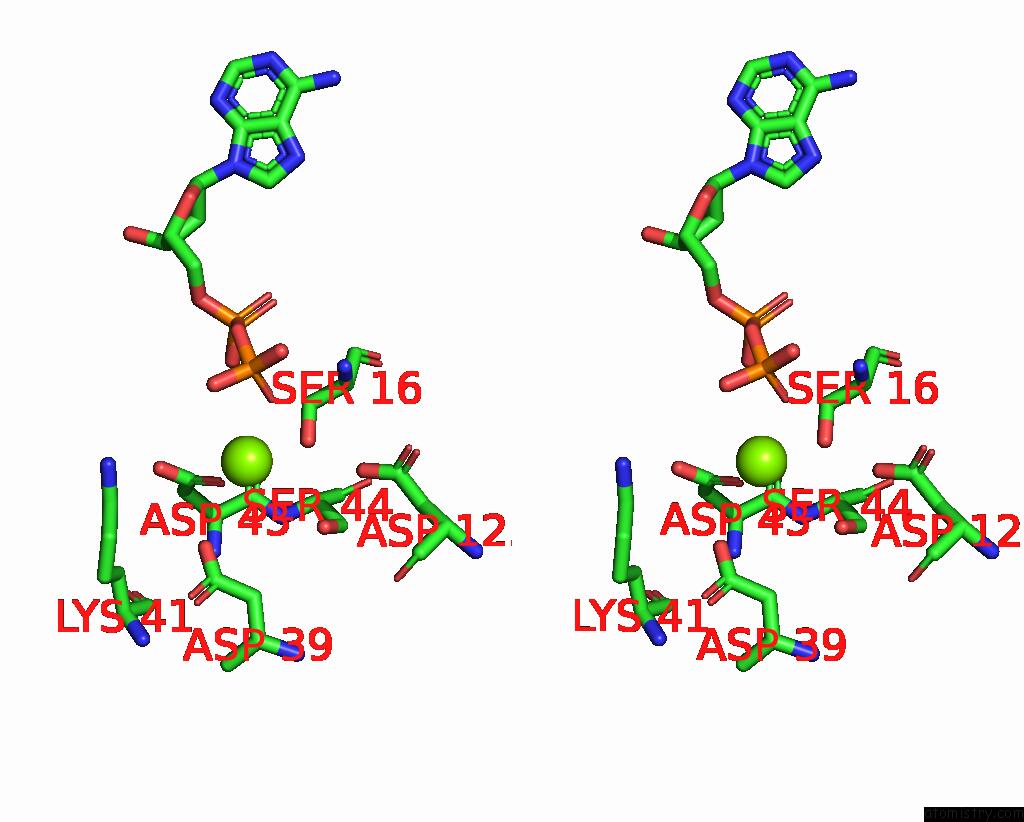
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 7uta
Go back to
Magnesium binding site 2 out
of 4 in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
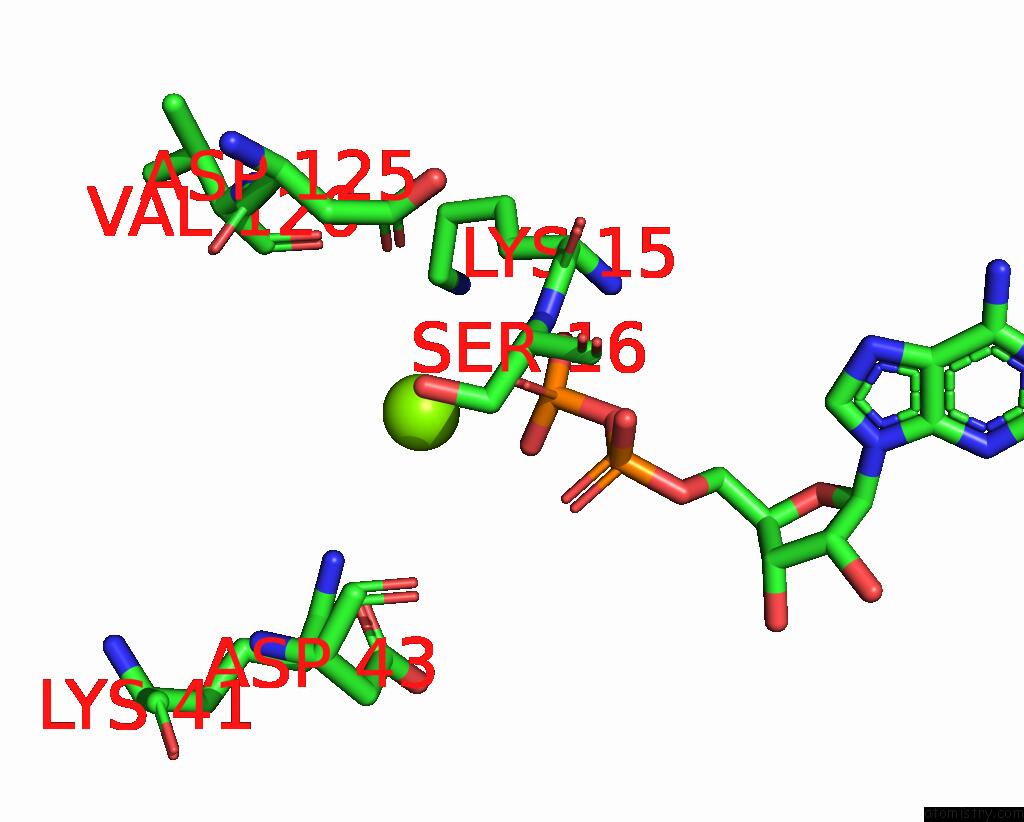
Mono view
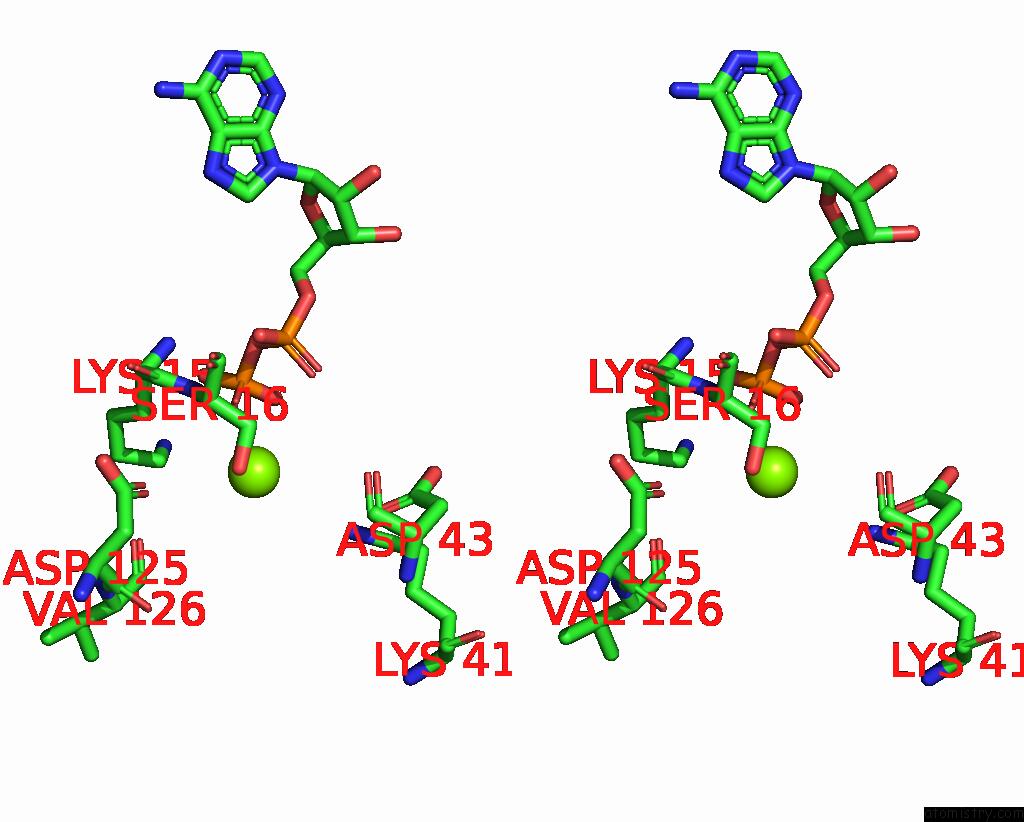
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction within 5.0Å range:
|
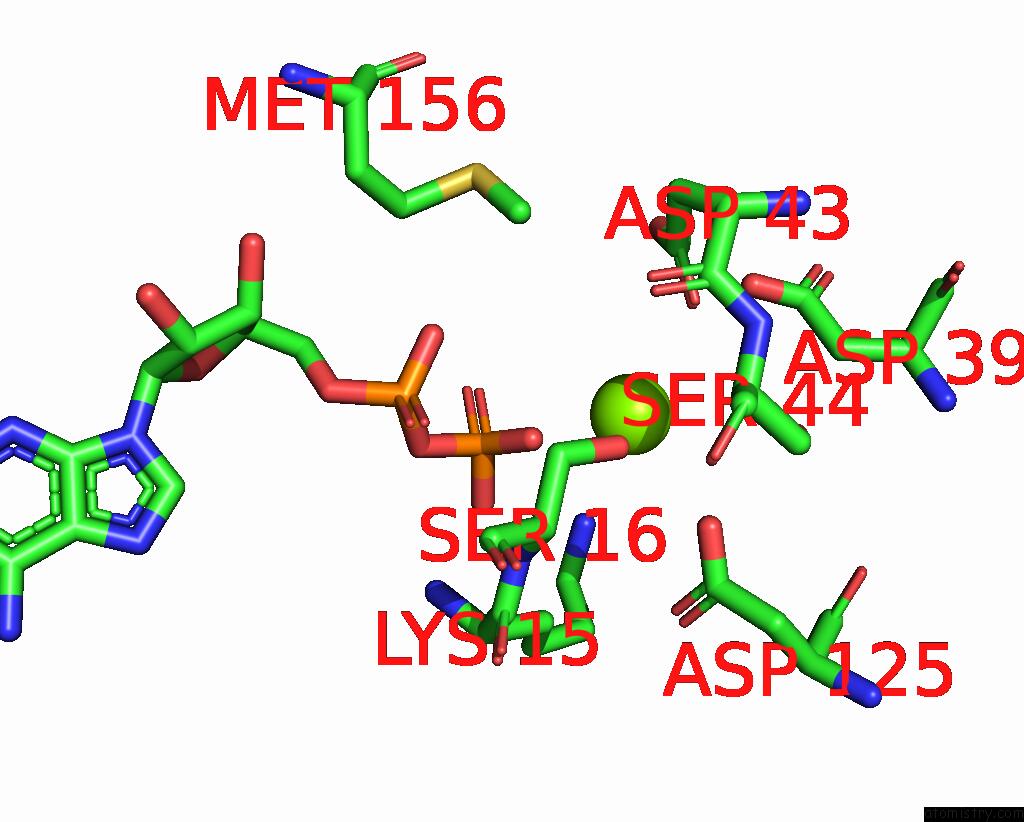
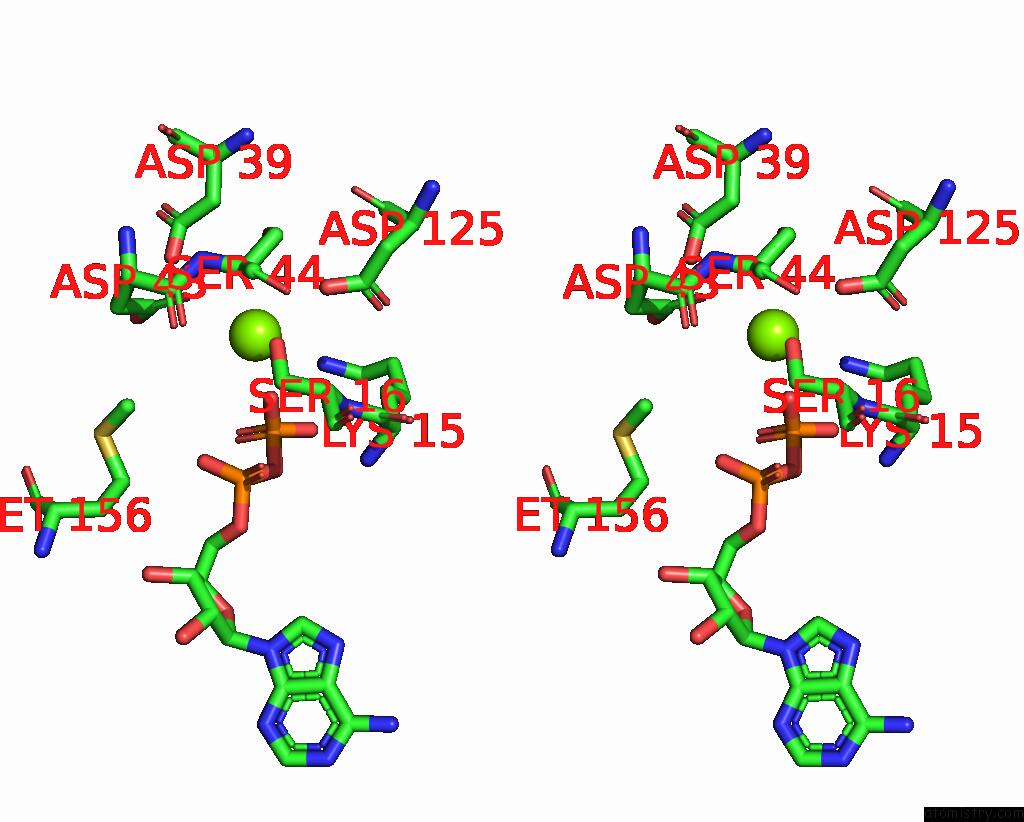
Magnesium binding site 3 out of 4 in 7uta
Go back to
Magnesium binding site 3 out
of 4 in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction within 5.0Å range:
|
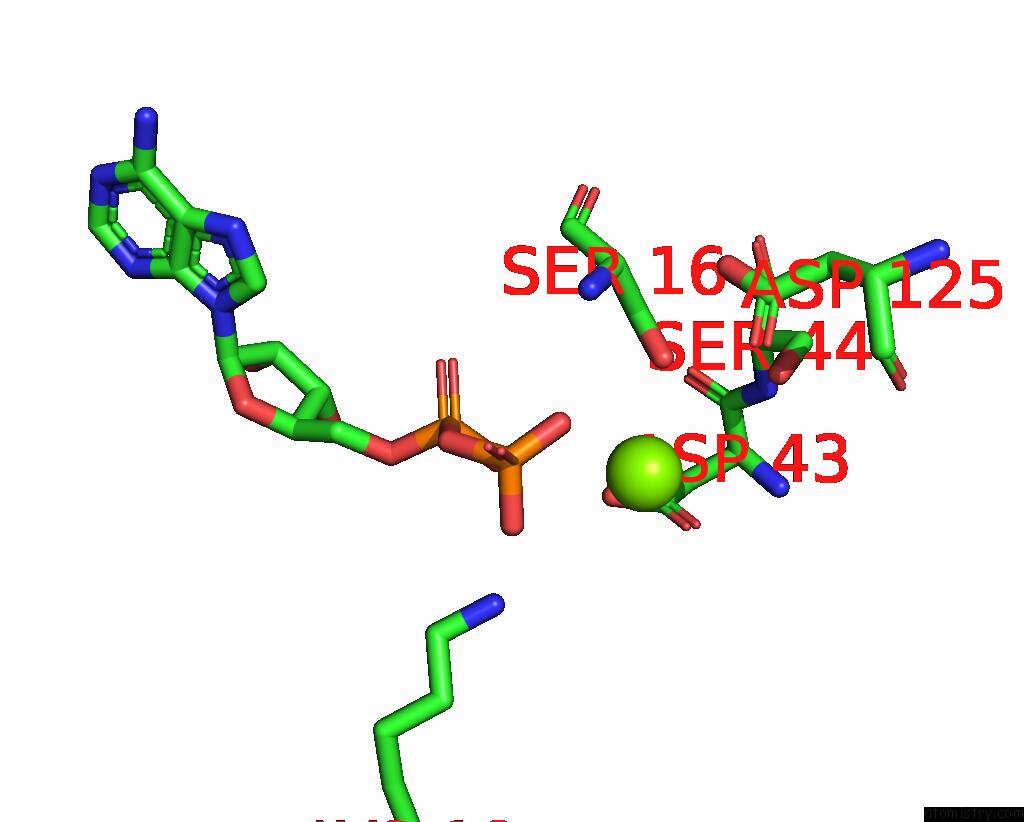
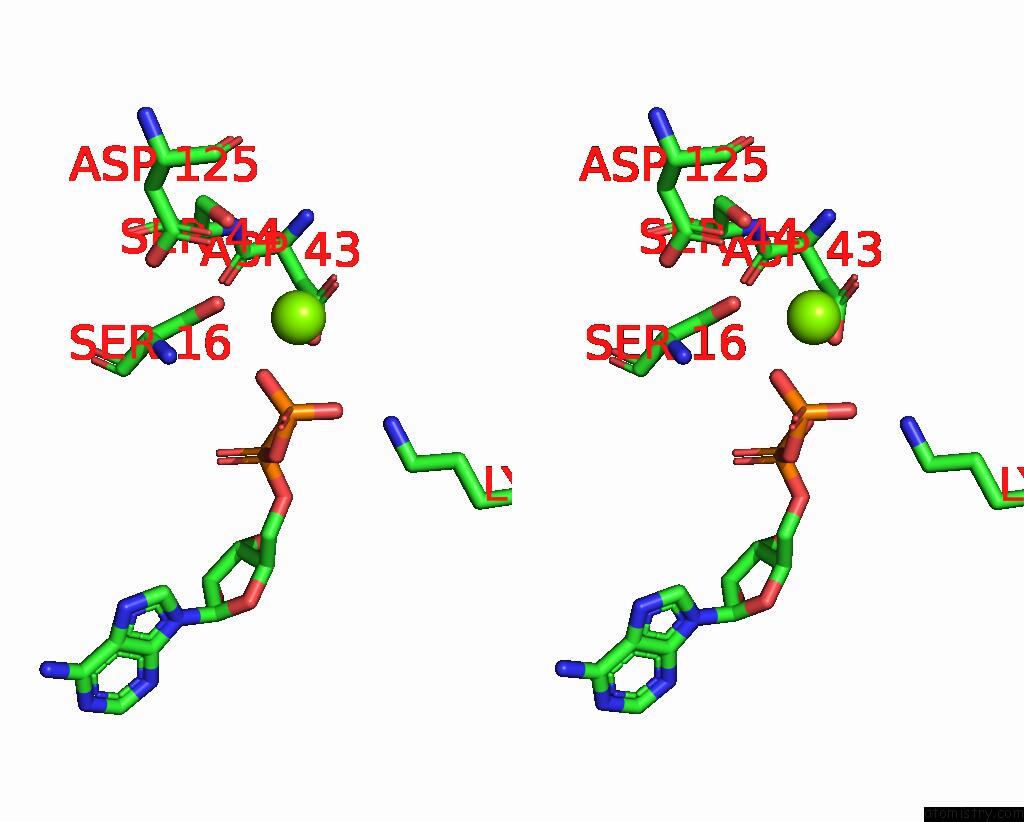
Magnesium binding site 4 out of 4 in 7uta
Go back to
Magnesium binding site 4 out
of 4 in the Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Cryoem Structure of Azotobacter Vinelandii Nitrogenase Complex (2:1 Fep:Mofep) Inhibited By Befx During Catalytic N2 Reduction within 5.0Å range:
|
Reference:
H.L.Rutledge,
B.D.Cook,
H.P.M.Nguyen,
M.A.Herzik Jr.,
F.A.Tezcan.
Structures of the Nitrogenase Complex Prepared Under Catalytic Turnover Conditions. Science V. 377 865 2022.
ISSN: ESSN 1095-9203
PubMed: 35901182
DOI: 10.1126/SCIENCE.ABQ7641
Page generated: Thu Oct 3 10:12:32 2024
ISSN: ESSN 1095-9203
PubMed: 35901182
DOI: 10.1126/SCIENCE.ABQ7641
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF