Magnesium »
PDB 7xld-7y41 »
7xzr »
Magnesium in PDB 7xzr: Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex
Enzymatic activity of Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex
All present enzymatic activity of Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex:
2.7.11.1;
2.7.11.1;
Protein crystallography data
The structure of Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex, PDB code: 7xzr
was solved by
K.Hamada,
A.A.Vinogradov,
Y.Zhang,
J.S.Chang,
H.Nishimura,
Y.Goto,
H.Onaka,
H.Suga,
K.Ogata,
T.Sengoku,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.34 / 2.26 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 53.11, 167.65, 53.71, 90, 108.5, 90 |
| R / Rfree (%) | 18.2 / 22.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex
(pdb code 7xzr). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex, PDB code: 7xzr:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex, PDB code: 7xzr:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 7xzr
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 7xzr
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex
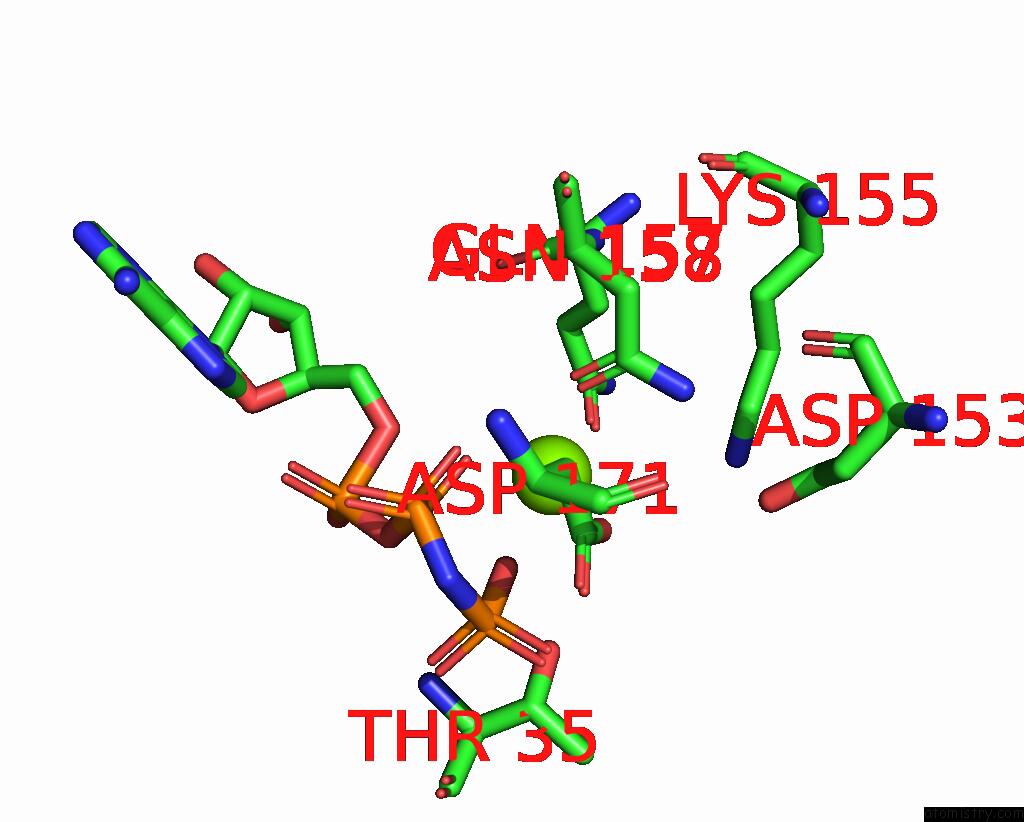
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Tnik-Amppnp-Thiopeptide TP15 Complex within 5.0Å range:
|
Reference:
A.A.Vinogradov,
Y.Zhang,
K.Hamada,
J.S.Chang,
C.Okada,
H.Nishimura,
N.Terasaka,
Y.Goto,
K.Ogata,
T.Sengoku,
H.Onaka,
H.Suga.
De Novo Discovery of Thiopeptide Pseudo-Natural Products Acting As Potent and Selective Tnik Kinase Inhibitors. J.Am.Chem.Soc. V. 144 20332 2022.
ISSN: ESSN 1520-5126
PubMed: 36282922
DOI: 10.1021/JACS.2C07937
Page generated: Thu Oct 3 13:24:55 2024
ISSN: ESSN 1520-5126
PubMed: 36282922
DOI: 10.1021/JACS.2C07937
Last articles
Cl in 7TNLCl in 7TNK
Cl in 7TNJ
Cl in 7TNH
Cl in 7TNF
Cl in 7TND
Cl in 7TN8
Cl in 7TNC
Cl in 7TN7
Cl in 7TN4