Magnesium »
PDB 7ygb-7yv1 »
7yry »
Magnesium in PDB 7yry: F1-Atpase of Acinetobacter Baumannii
Enzymatic activity of F1-Atpase of Acinetobacter Baumannii
All present enzymatic activity of F1-Atpase of Acinetobacter Baumannii:
7.1.2.2;
7.1.2.2;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the F1-Atpase of Acinetobacter Baumannii
(pdb code 7yry). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the F1-Atpase of Acinetobacter Baumannii, PDB code: 7yry:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the F1-Atpase of Acinetobacter Baumannii, PDB code: 7yry:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 7yry
Go back to
Magnesium binding site 1 out
of 4 in the F1-Atpase of Acinetobacter Baumannii
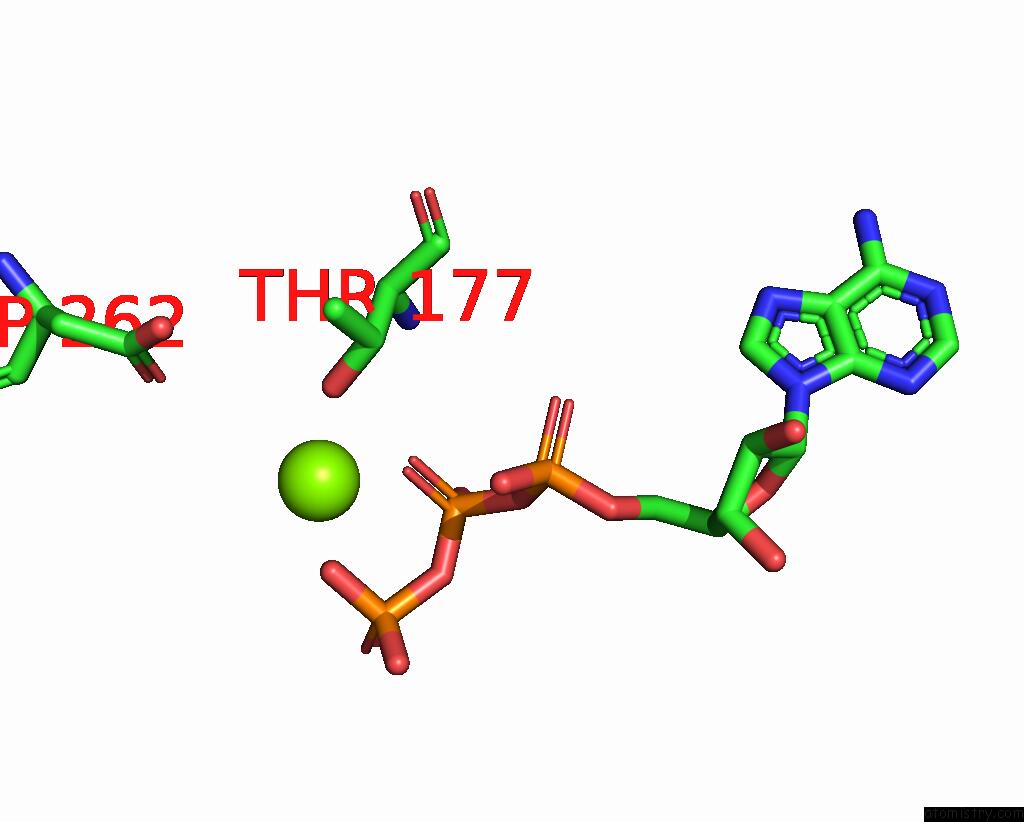
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of F1-Atpase of Acinetobacter Baumannii within 5.0Å range:
|
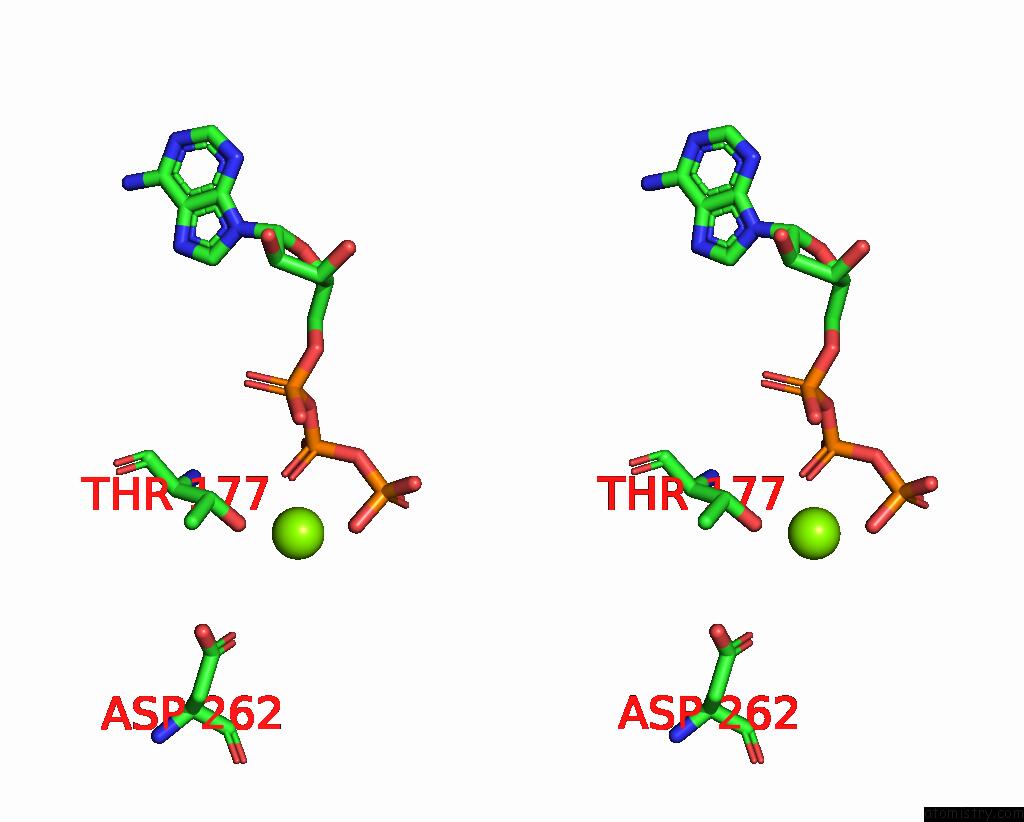
Magnesium binding site 2 out of 4 in 7yry
Go back to
Magnesium binding site 2 out
of 4 in the F1-Atpase of Acinetobacter Baumannii

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of F1-Atpase of Acinetobacter Baumannii within 5.0Å range:
|
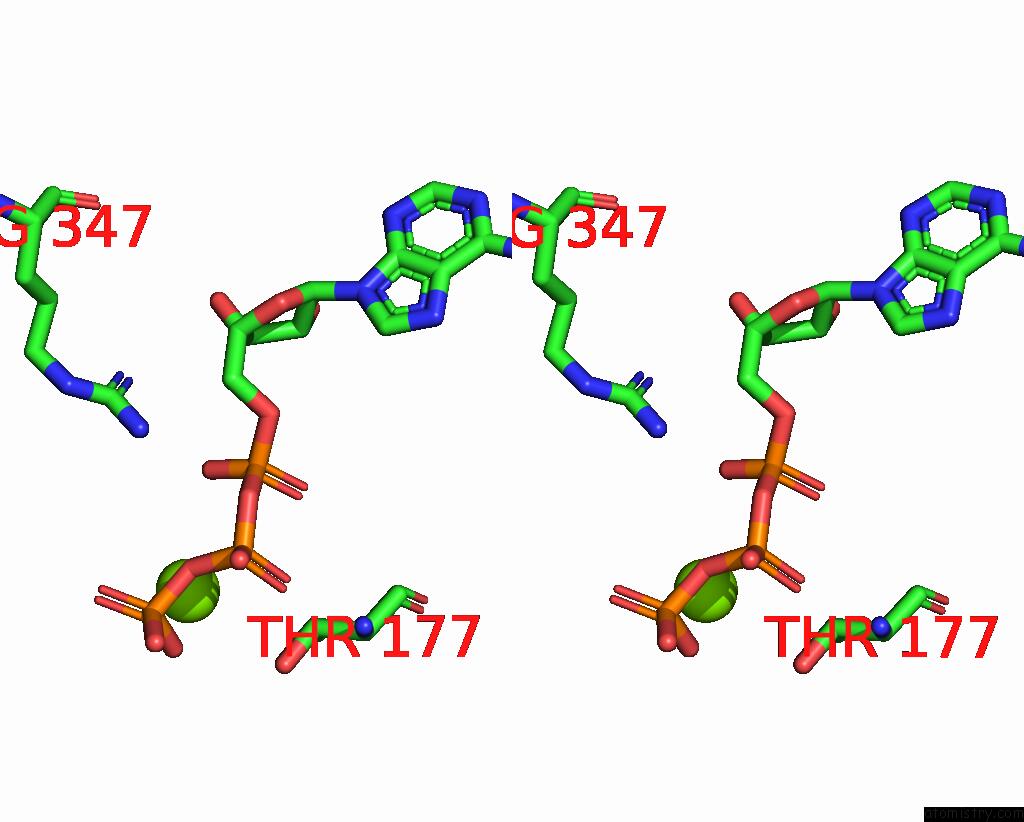
Magnesium binding site 3 out of 4 in 7yry
Go back to
Magnesium binding site 3 out
of 4 in the F1-Atpase of Acinetobacter Baumannii
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of F1-Atpase of Acinetobacter Baumannii within 5.0Å range:
|
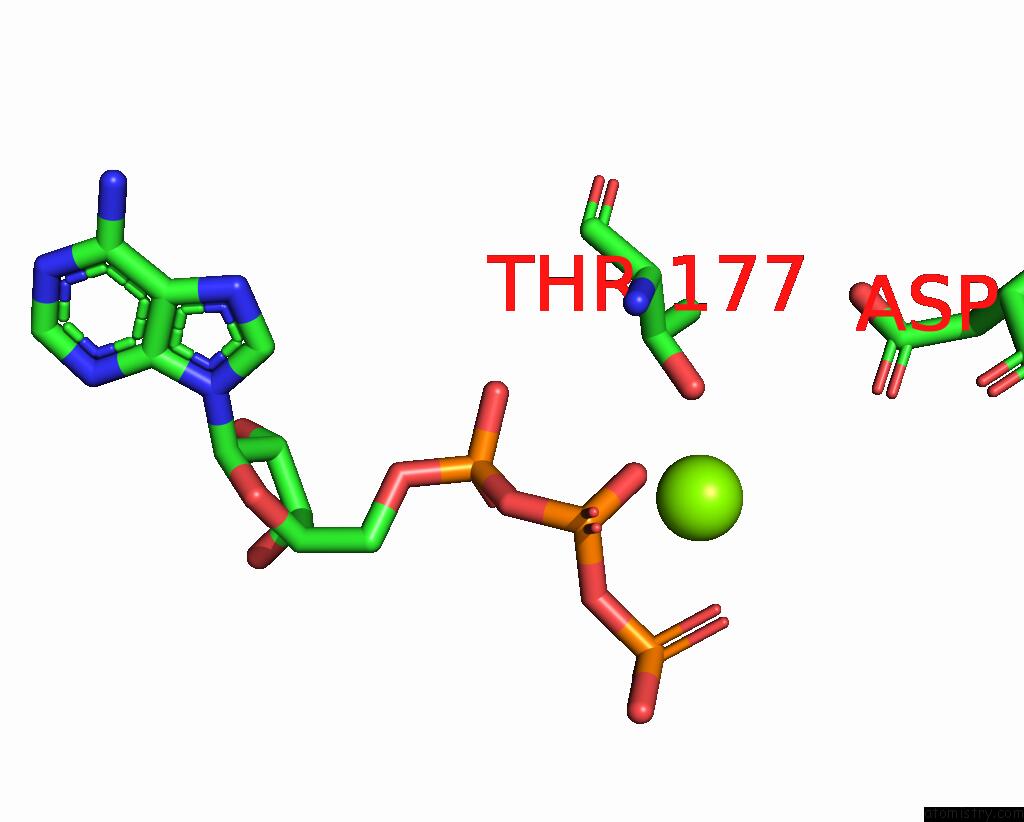
Magnesium binding site 4 out of 4 in 7yry
Go back to
Magnesium binding site 4 out
of 4 in the F1-Atpase of Acinetobacter Baumannii
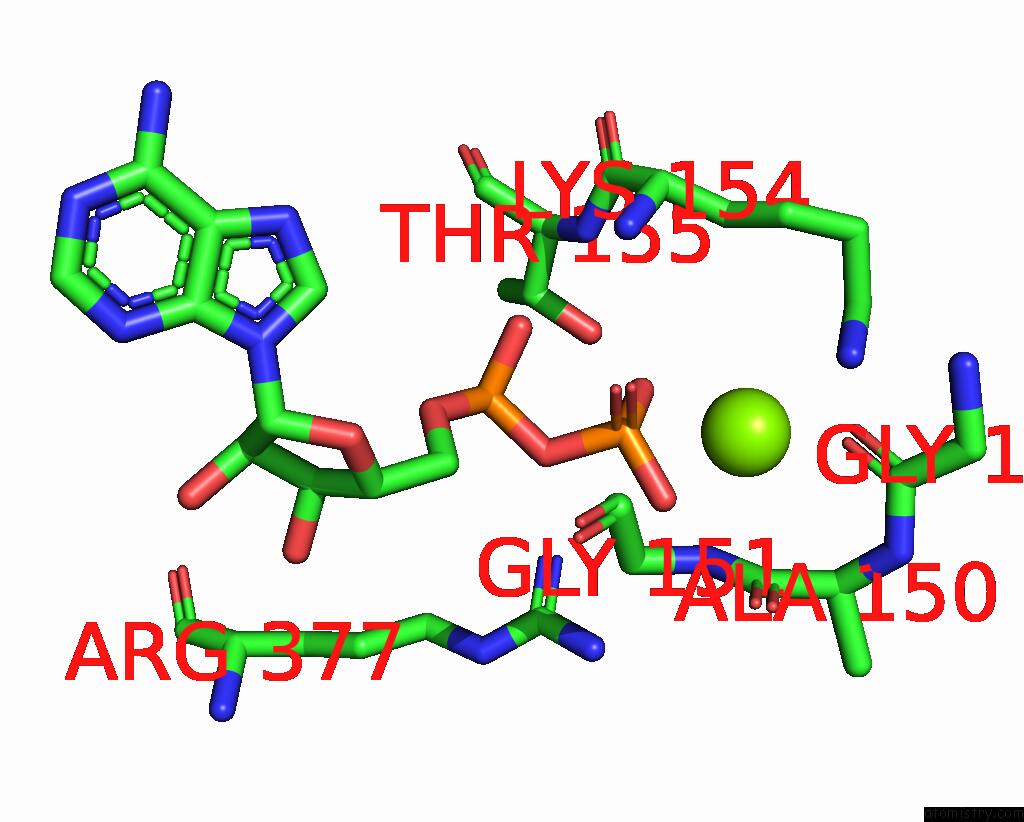
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of F1-Atpase of Acinetobacter Baumannii within 5.0Å range:
|
Reference:
W.G.Saw,
K.C.M.Le,
J.Shi,
J.H.M.Kwek,
C.F.Wong,
P.Ragunathan,
T.C.Fong,
V.Muller,
G.Grueber.
Atomic Insights of An Up and Down Conformation of the Acinetobacter Baumannii F1-Atpase Subunit Epsilon and Deciphering the Residues Critical For Atp Hydrolysis Inhibition and Atp Synthesis. Faseb J. V. 37 2023.
ISSN: ESSN 1530-6860
DOI: 10.1096/FJ.202300175RR
Page generated: Thu Oct 3 15:40:04 2024
ISSN: ESSN 1530-6860
DOI: 10.1096/FJ.202300175RR
Last articles
Ca in 2VC2Ca in 2VCP
Ca in 2VBO
Ca in 2VCC
Ca in 2VCA
Ca in 2VCB
Ca in 2VC9
Ca in 2VBN
Ca in 2VAY
Ca in 2VB6