Magnesium »
PDB 7zcu-7zmj »
7zei »
Magnesium in PDB 7zei: Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
Protein crystallography data
The structure of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A, PDB code: 7zei
was solved by
M.Baudrexl,
T.Fida,
B.Berk,
W.Schwarz,
V.V.Zverlov,
M.Groll,
W.Liebl,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 1.70 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 91.09, 108.74, 251.27, 90, 90, 90 |
| R / Rfree (%) | 12.6 / 15.3 |
Other elements in 7zei:
The structure of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A also contains other interesting chemical elements:
| Calcium | (Ca) | 6 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
(pdb code 7zei). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A, PDB code: 7zei:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A, PDB code: 7zei:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 7zei
Go back to
Magnesium binding site 1 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
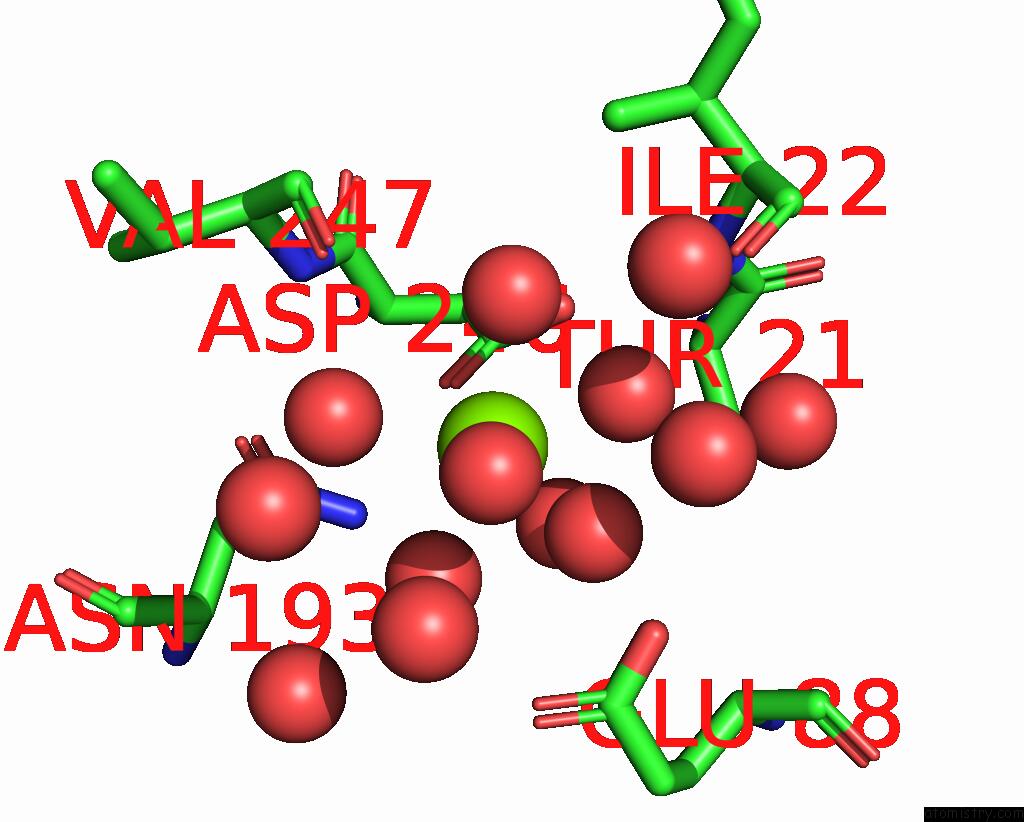
Mono view
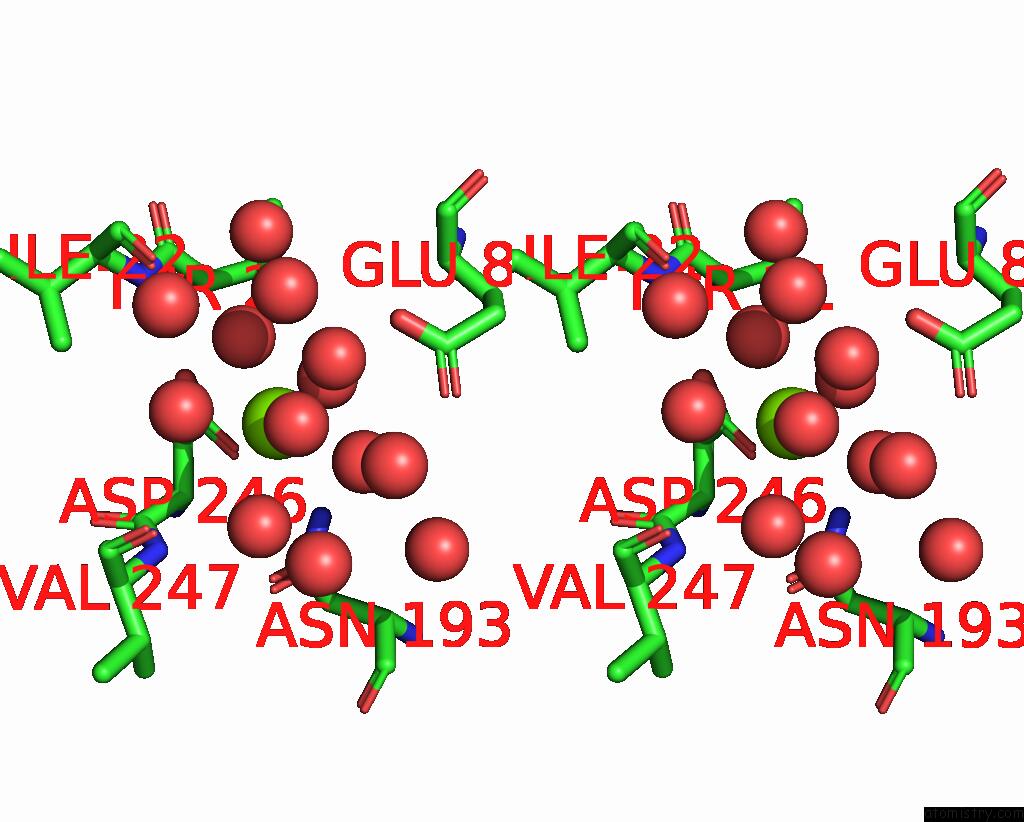
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 7zei
Go back to
Magnesium binding site 2 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
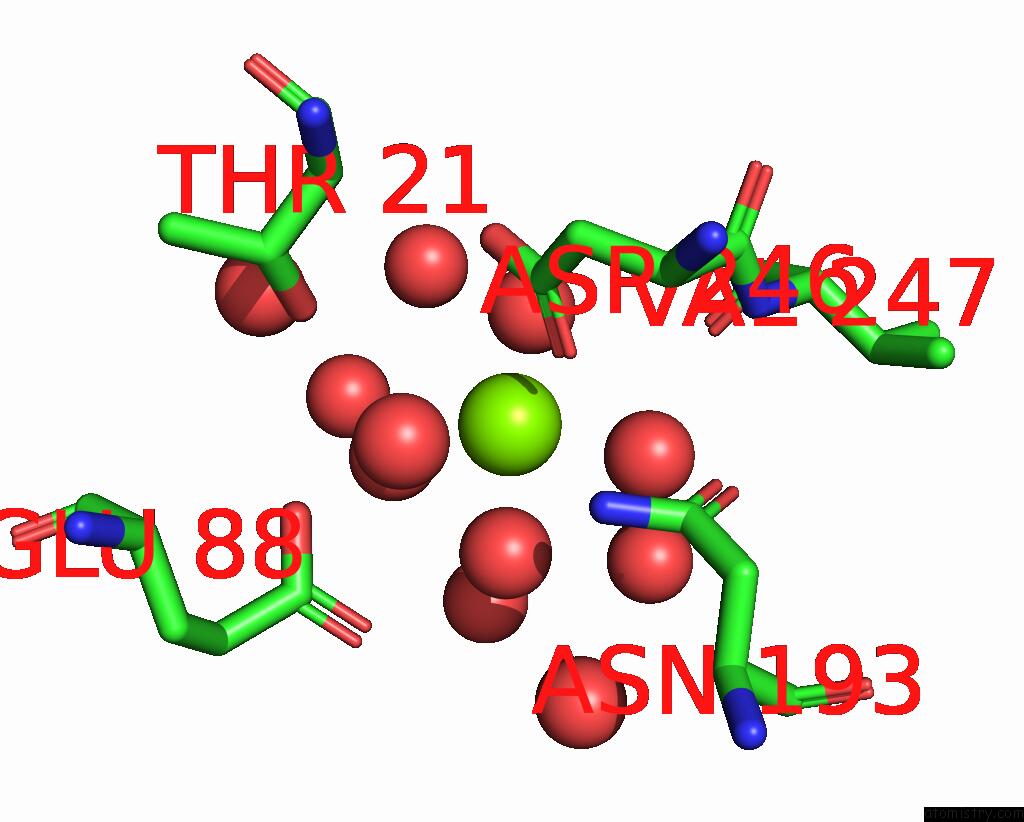
Mono view
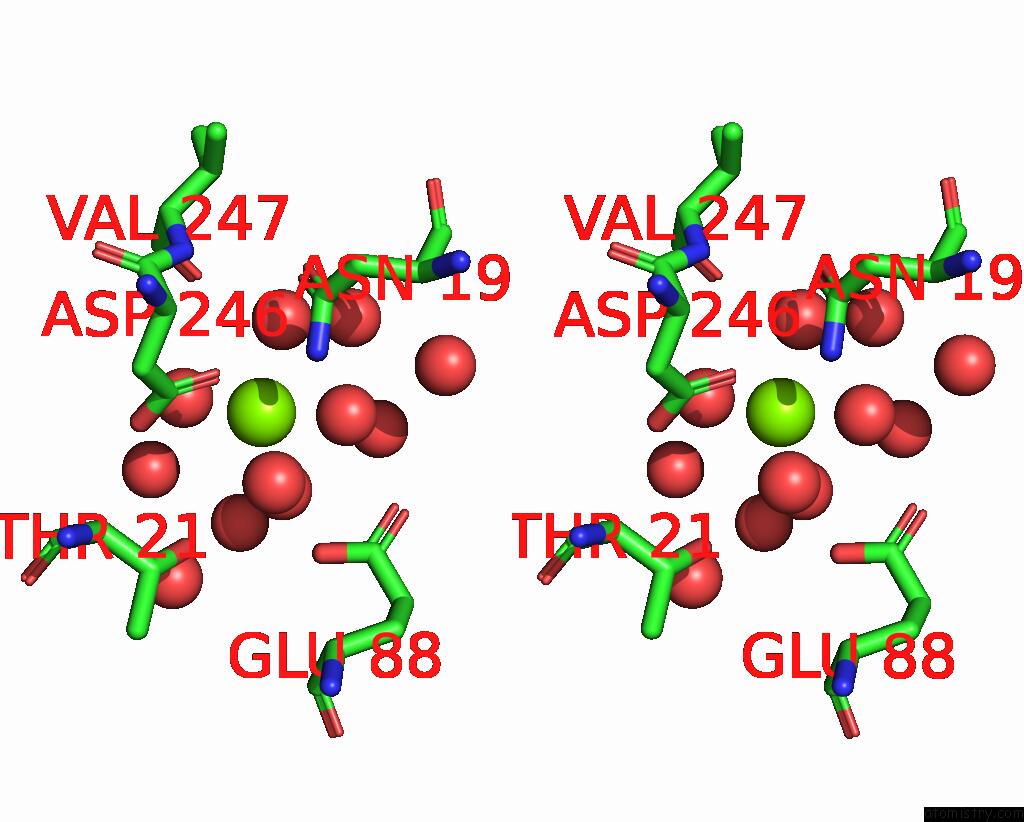
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 7zei
Go back to
Magnesium binding site 3 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
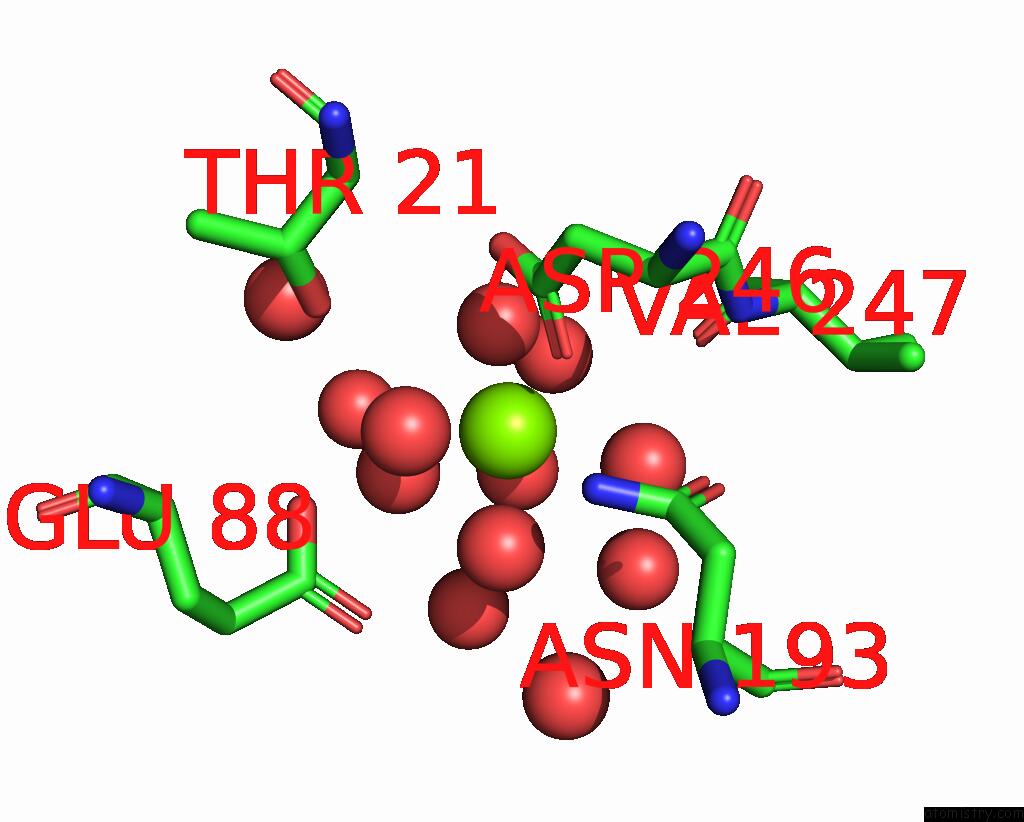
Mono view
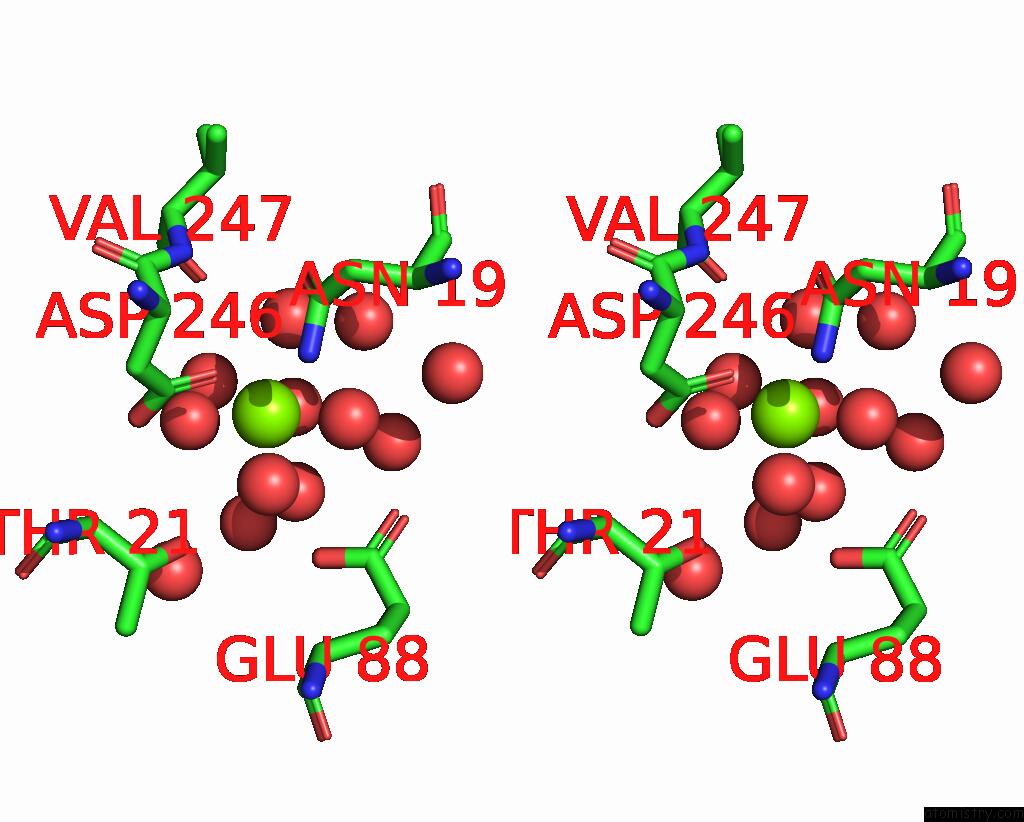
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 7zei
Go back to
Magnesium binding site 4 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
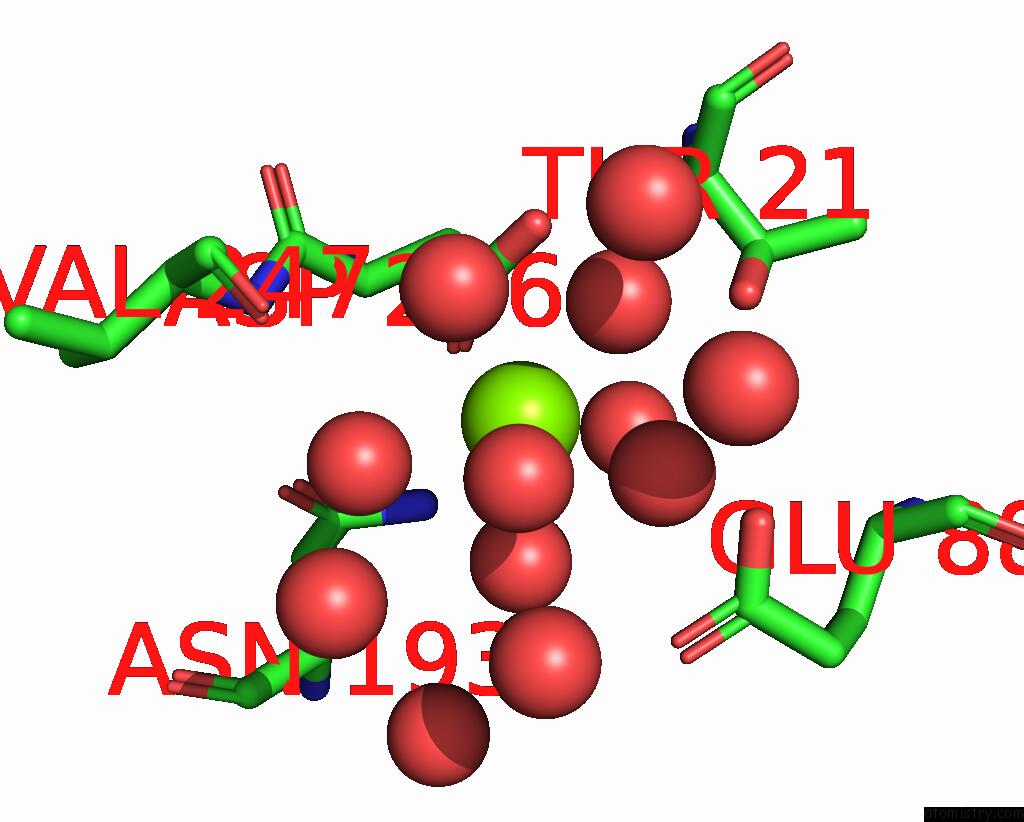
Mono view
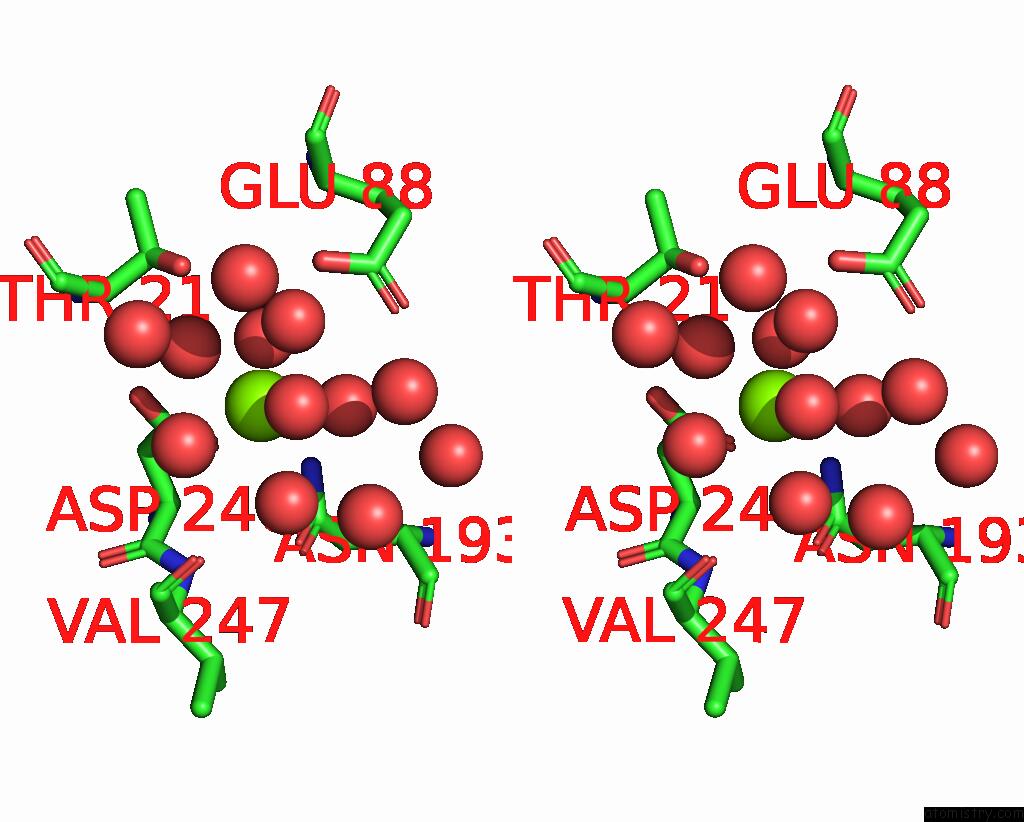
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 7zei
Go back to
Magnesium binding site 5 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
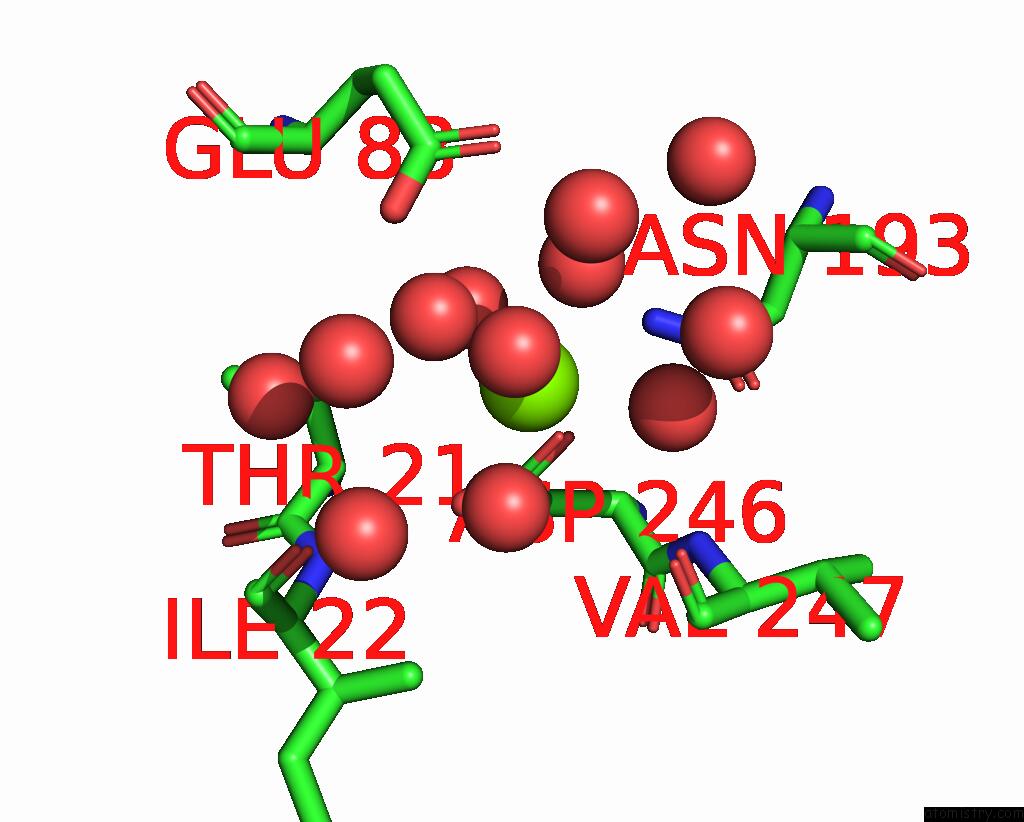
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 7zei
Go back to
Magnesium binding site 6 out
of 6 in the Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A
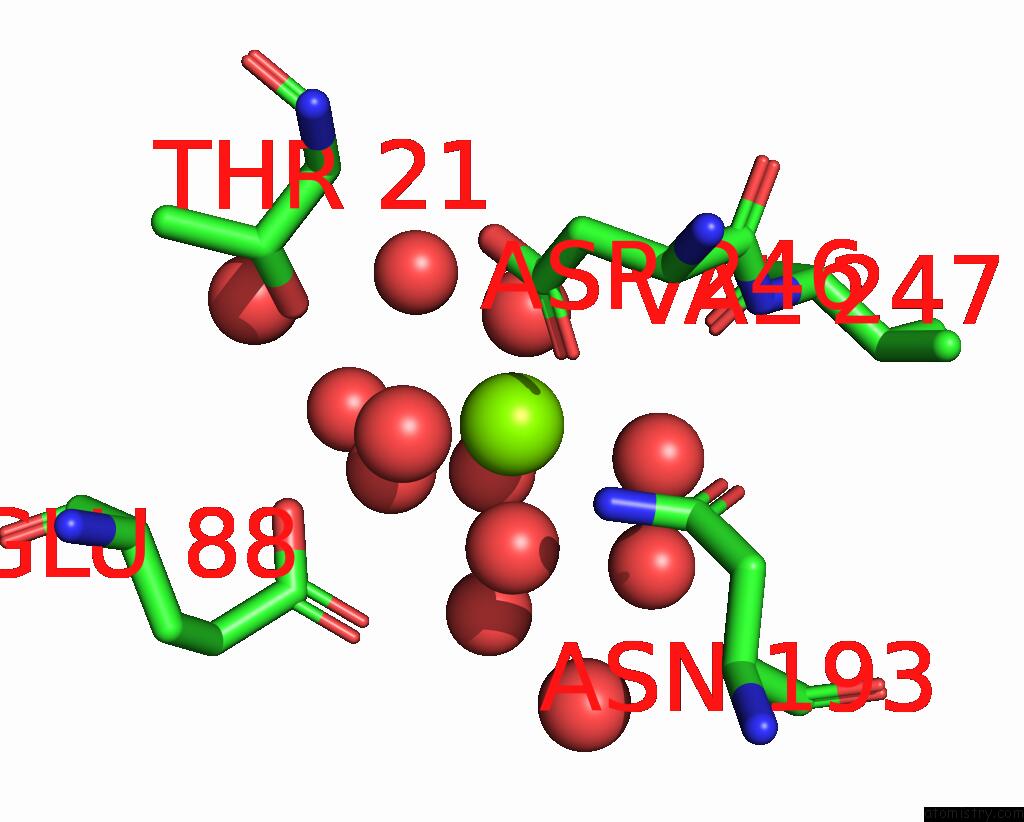
Mono view
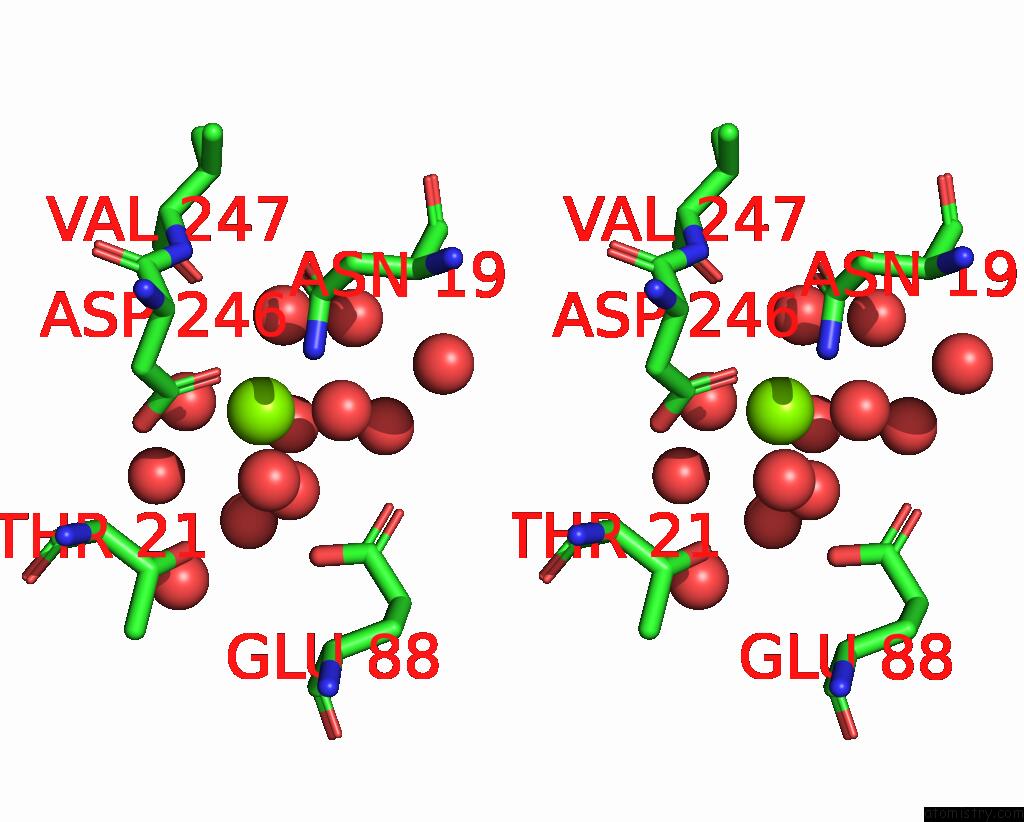
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Thermostable GH159 Glycoside Hydrolase From Caldicellulosiruptor at 1.7 A within 5.0Å range:
|
Reference:
M.Baudrexl,
T.Fida,
B.Berk,
W.H.Schwarz,
V.V.Zverlov,
M.Groll,
W.Liebl.
Biochemical and Structural Characterization of Thermostable GH159 Glycoside Hydrolases Exhibiting Alpha-L-Arabinofuranosidase Activity. Front Mol Biosci V. 9 07439 2022.
ISSN: ESSN 2296-889X
PubMed: 35847984
DOI: 10.3389/FMOLB.2022.907439
Page generated: Thu Oct 3 16:45:07 2024
ISSN: ESSN 2296-889X
PubMed: 35847984
DOI: 10.3389/FMOLB.2022.907439
Last articles
Cl in 8A1HCl in 8A1B
Cl in 8A0V
Cl in 8A0U
Cl in 8A0Z
Cl in 8A0S
Cl in 8A0R
Cl in 8A0Q
Cl in 7ZZS
Cl in 8A0P