Magnesium »
PDB 8cqx-8d71 »
8d32 »
Magnesium in PDB 8d32: Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi
Enzymatic activity of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi
All present enzymatic activity of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi:
2.5.1.17;
2.5.1.17;
Protein crystallography data
The structure of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi, PDB code: 8d32
was solved by
R.N.Mascarenhas,
M.Ruetz,
M.Koutmos,
R.Banerjee,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.05 / 1.85 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.618, 62.618, 266.997, 90, 90, 120 |
| R / Rfree (%) | 21.9 / 24.6 |
Other elements in 8d32:
The structure of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi also contains other interesting chemical elements:
| Rhodium | (Rh) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi
(pdb code 8d32). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi, PDB code: 8d32:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi, PDB code: 8d32:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8d32
Go back to
Magnesium binding site 1 out
of 2 in the Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi
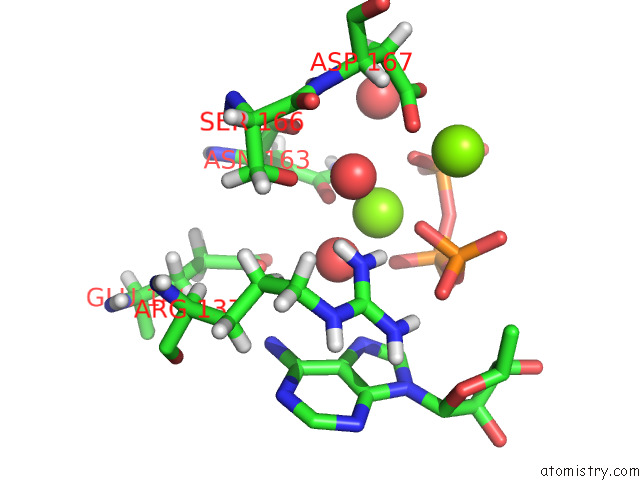
Mono view
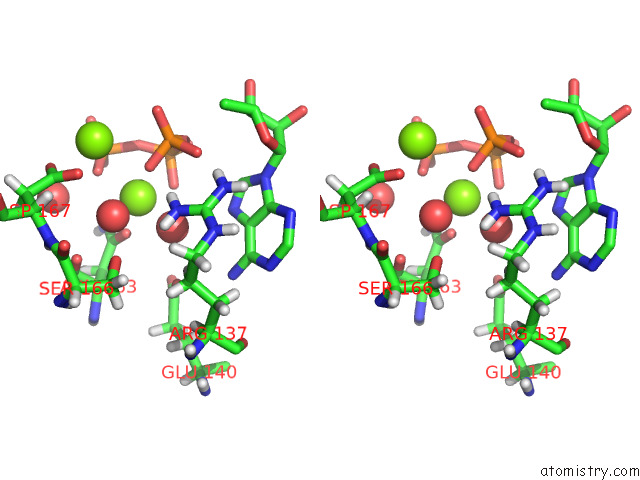
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8d32
Go back to
Magnesium binding site 2 out
of 2 in the Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi
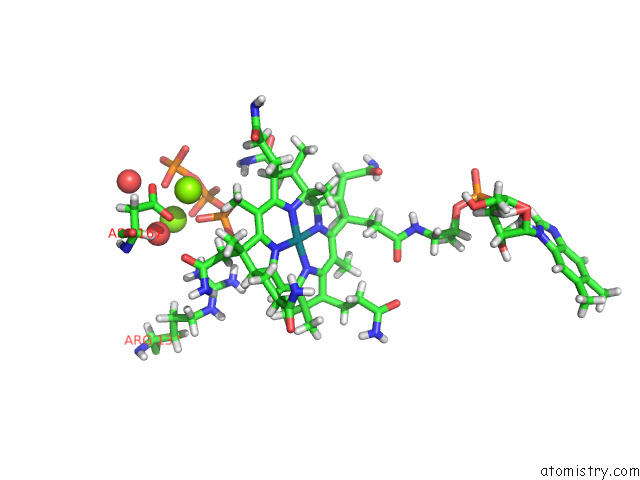
Mono view
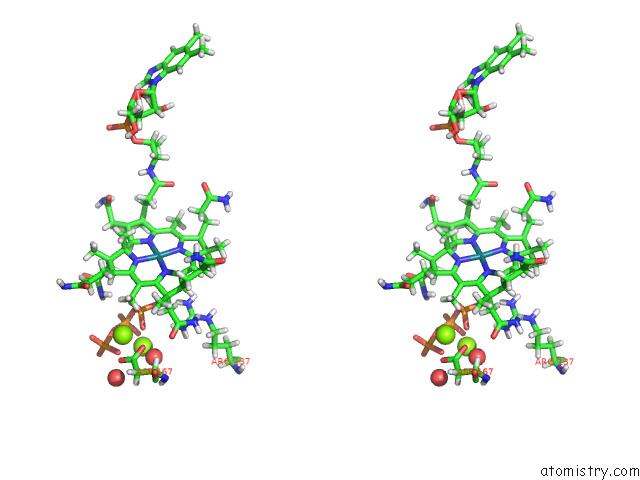
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Mycobacterium Tuberculosis Pduo-Type Atp:Cobalamin Adenosyltransferase Bound to 5-Deoxyadenosylrhodibalamin and Pppi within 5.0Å range:
|
Reference:
M.Ruetz,
R.N.Mascarenhas,
M.Koutmos,
B.Krautler,
R.Banerjee.
A Noble Substitution Leads to the Cofactor Mimicry By Rhodibalamin To Be Published.
Page generated: Thu Oct 3 23:37:03 2024
Last articles
Cl in 7UP4Cl in 7UOS
Cl in 7UPI
Cl in 7UMO
Cl in 7UOD
Cl in 7UN0
Cl in 7UOQ
Cl in 7UOC
Cl in 7UNO
Cl in 7UNN