Magnesium »
PDB 8gwk-8h5m »
8h47 »
Magnesium in PDB 8h47: Blasnase-T13A/P55F
Protein crystallography data
The structure of Blasnase-T13A/P55F, PDB code: 8h47
was solved by
F.Lu,
W.Wang,
H.Chi,
T.Ran,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.98 / 1.90 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 92.513, 92.513, 230.783, 90, 90, 90 |
| R / Rfree (%) | 19.2 / 20.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Blasnase-T13A/P55F
(pdb code 8h47). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Blasnase-T13A/P55F, PDB code: 8h47:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Blasnase-T13A/P55F, PDB code: 8h47:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 8h47
Go back to
Magnesium binding site 1 out
of 4 in the Blasnase-T13A/P55F

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Blasnase-T13A/P55F within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 8h47
Go back to
Magnesium binding site 2 out
of 4 in the Blasnase-T13A/P55F
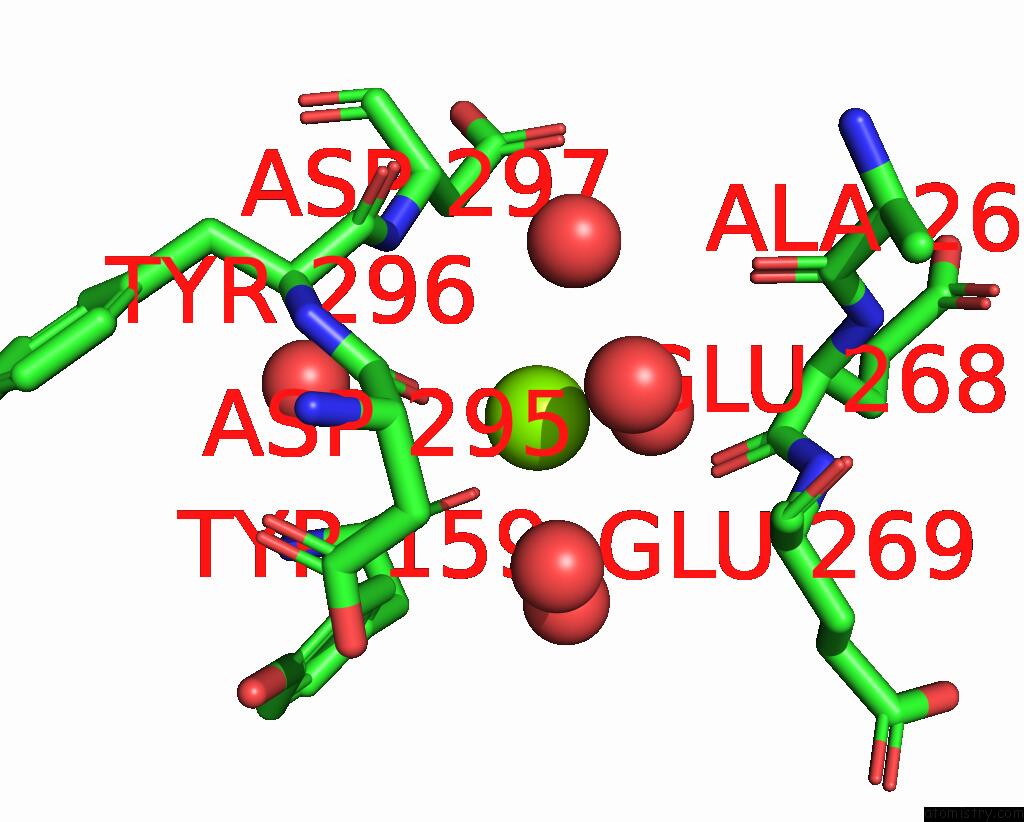
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Blasnase-T13A/P55F within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 8h47
Go back to
Magnesium binding site 3 out
of 4 in the Blasnase-T13A/P55F

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Blasnase-T13A/P55F within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 8h47
Go back to
Magnesium binding site 4 out
of 4 in the Blasnase-T13A/P55F
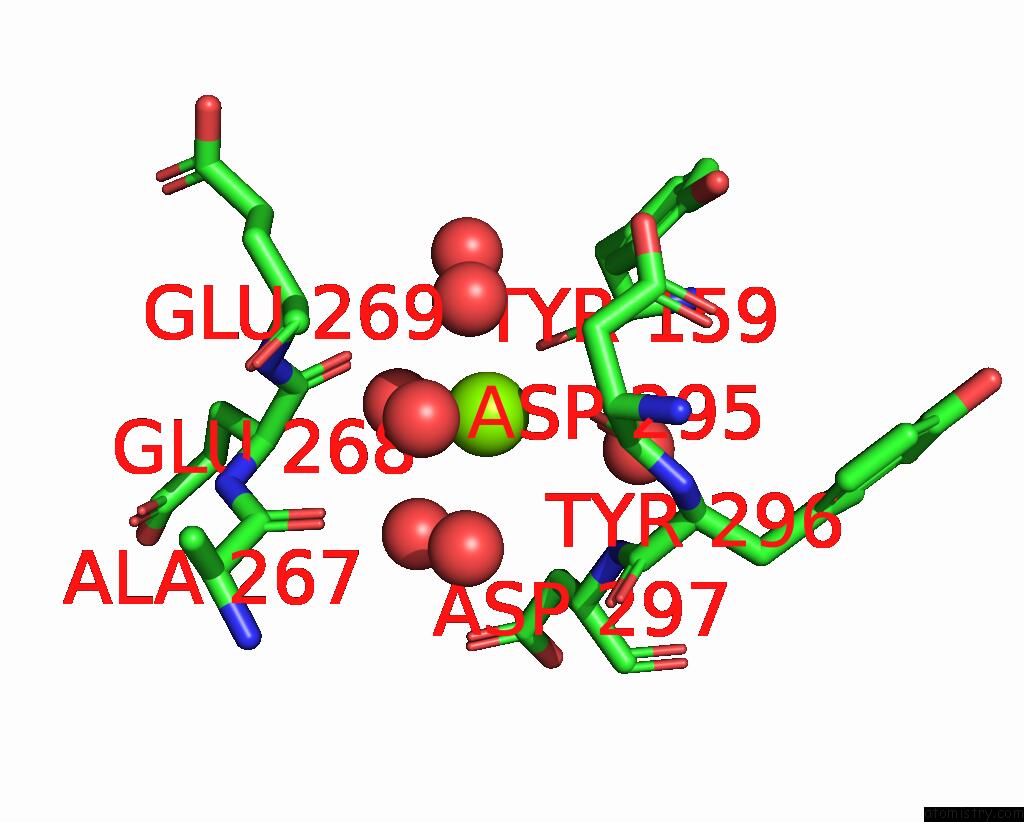
Mono view
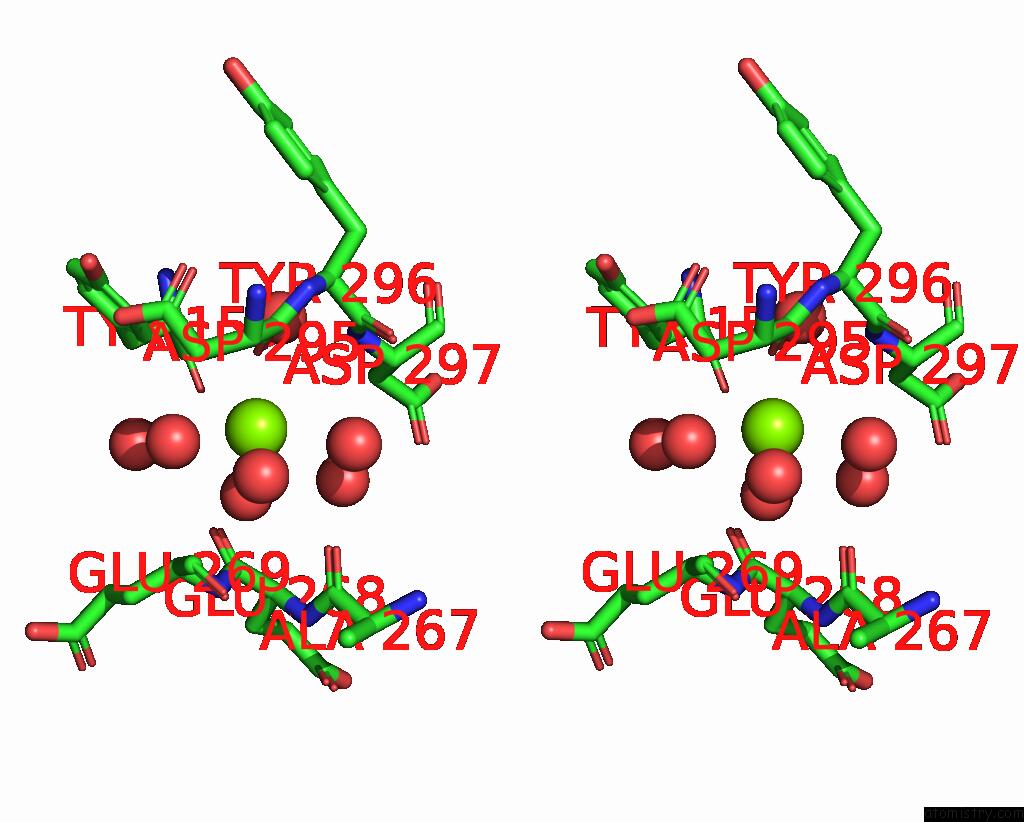
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Blasnase-T13A/P55F within 5.0Å range:
|
Reference:
F.Lu,
W.Wang,
H.Chi,
T.Ran.
Structure-Based Rational Design of Bacillus Licheniformis L-Asparaginase with Low/No D-Asparaginase Activity For A Safer Enzyme To Be Published.
Page generated: Fri Oct 4 04:18:48 2024
Last articles
F in 7GP0F in 7GPG
F in 7GO5
F in 7GPI
F in 7GPA
F in 7GP8
F in 7GOR
F in 7GOV
F in 7GON
F in 7GOB