Magnesium »
PDB 8h5y-8hh8 »
8hbh »
Magnesium in PDB 8hbh: Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom
Enzymatic activity of Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom
All present enzymatic activity of Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom:
4.6.1.2;
4.6.1.2;
Other elements in 8hbh:
The structure of Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom also contains other interesting chemical elements:
| Iron | (Fe) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom
(pdb code 8hbh). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom, PDB code: 8hbh:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom, PDB code: 8hbh:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8hbh
Go back to
Magnesium binding site 1 out
of 2 in the Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom

Mono view
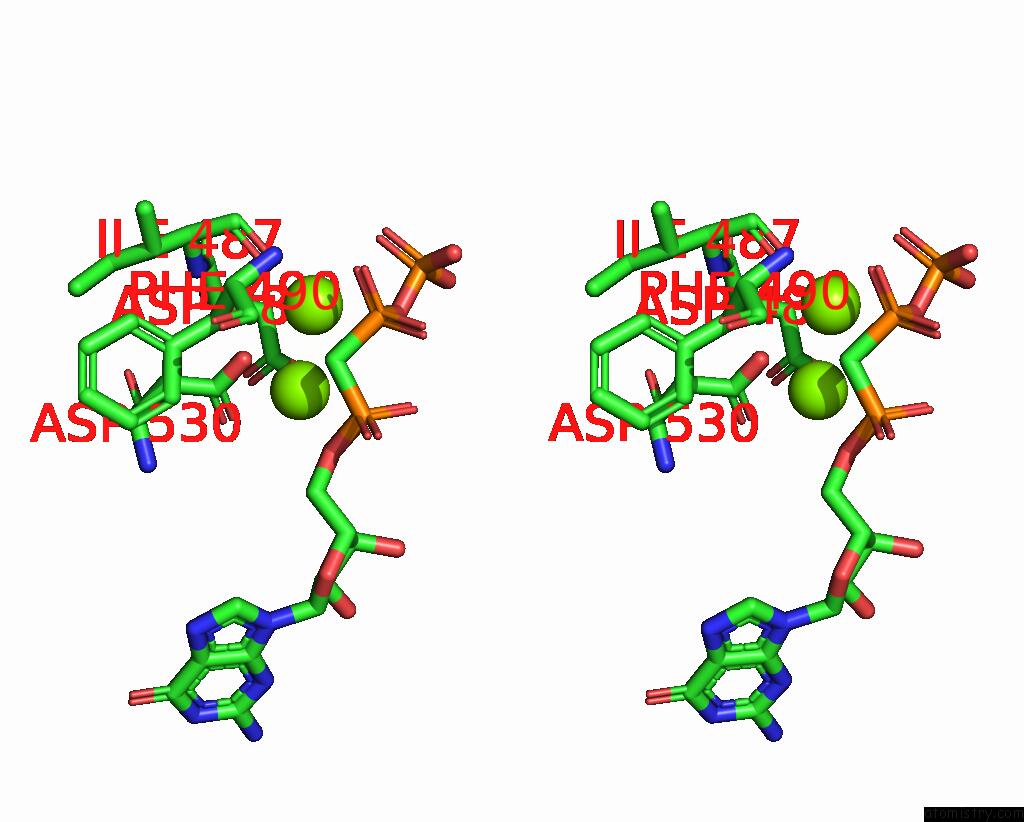
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8hbh
Go back to
Magnesium binding site 2 out
of 2 in the Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom
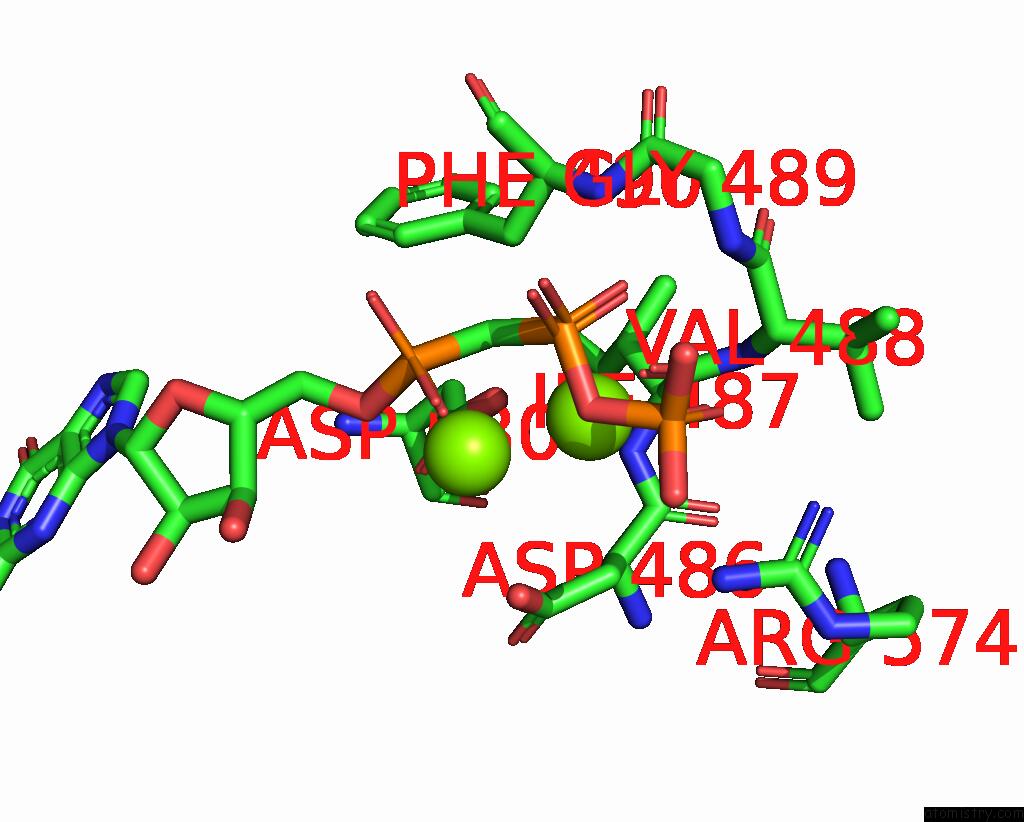
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Human Soluble Guanylate Cyclase in the No-Activated State at 3.1 Angstrom within 5.0Å range:
|
Reference:
R.Liu,
Y.Kang,
L.Chen.
No Binds to the Distal Site of Haem in the Fully Activated Soluble Guanylate Cyclase. Nitric Oxide V.-135 17 2023.
ISSN: ESSN 1089-8611
PubMed: 36972843
DOI: 10.1016/J.NIOX.2023.03.002
Page generated: Fri Oct 4 04:47:18 2024
ISSN: ESSN 1089-8611
PubMed: 36972843
DOI: 10.1016/J.NIOX.2023.03.002
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO