Magnesium »
PDB 8h5y-8hh8 »
8hh1 »
Magnesium in PDB 8hh1: FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
Enzymatic activity of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
All present enzymatic activity of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp:
7.1.2.2;
7.1.2.2;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
(pdb code 8hh1). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 5 binding sites of Magnesium where determined in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp, PDB code: 8hh1:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Magnesium where determined in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp, PDB code: 8hh1:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5;
Magnesium binding site 1 out of 5 in 8hh1
Go back to
Magnesium binding site 1 out
of 5 in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
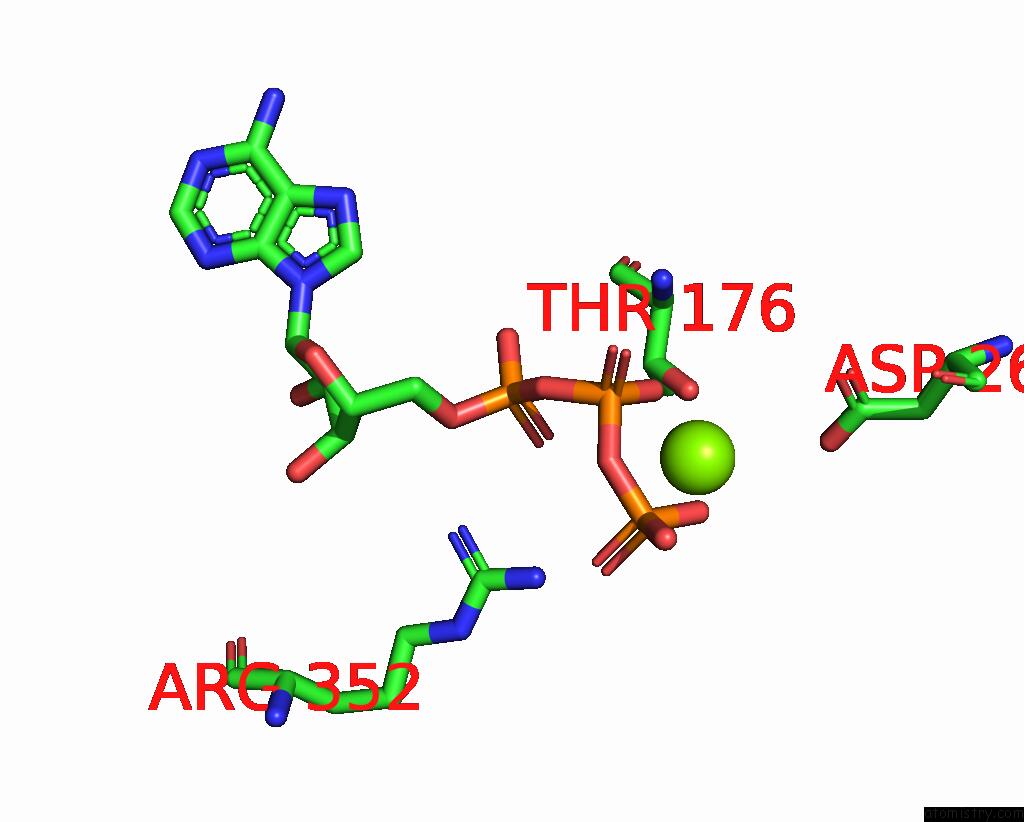
Mono view
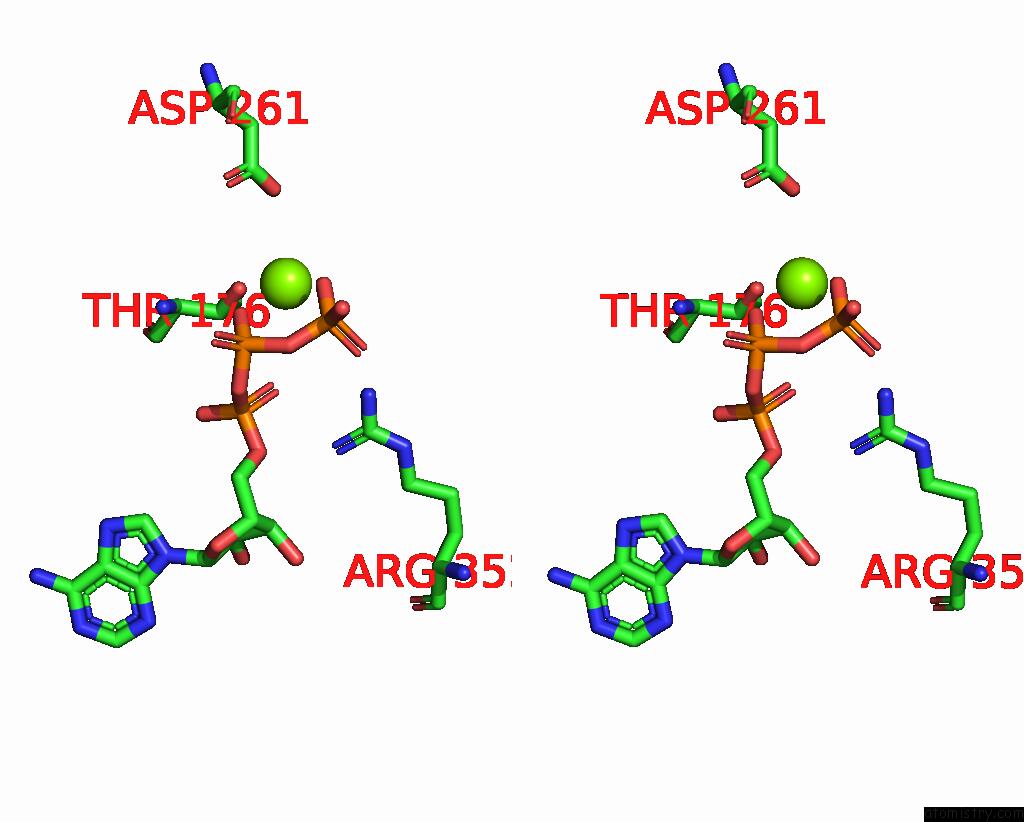
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp within 5.0Å range:
|
Magnesium binding site 2 out of 5 in 8hh1
Go back to
Magnesium binding site 2 out
of 5 in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
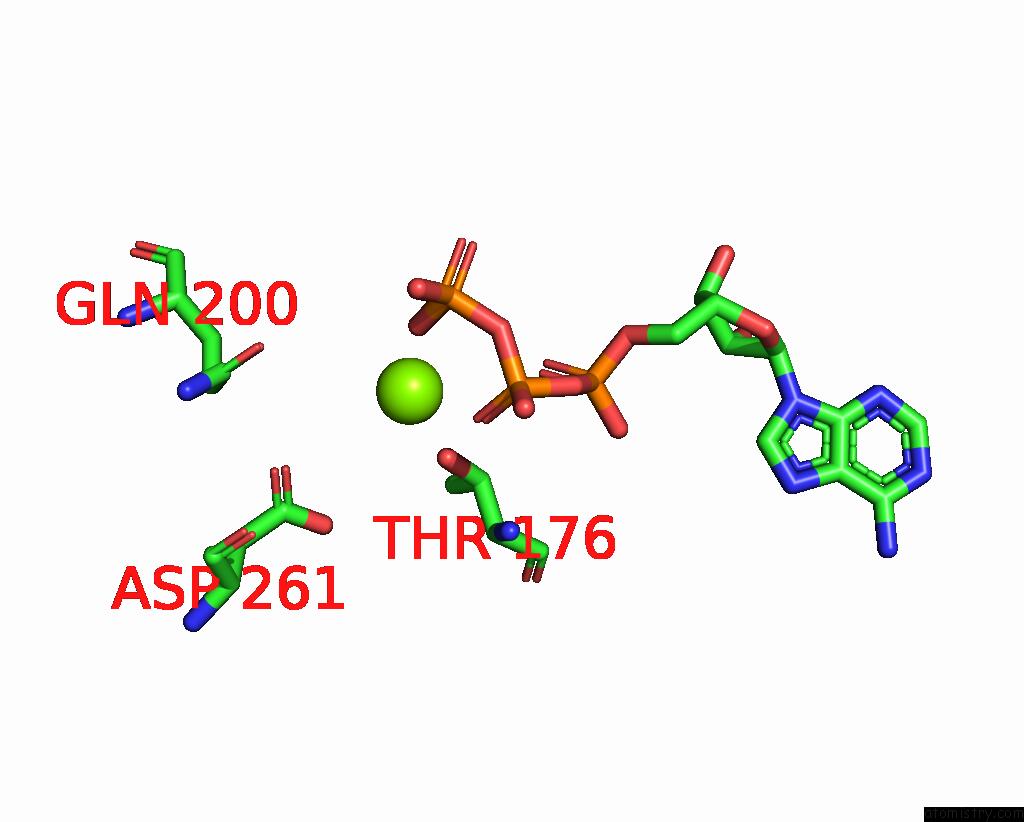
Mono view
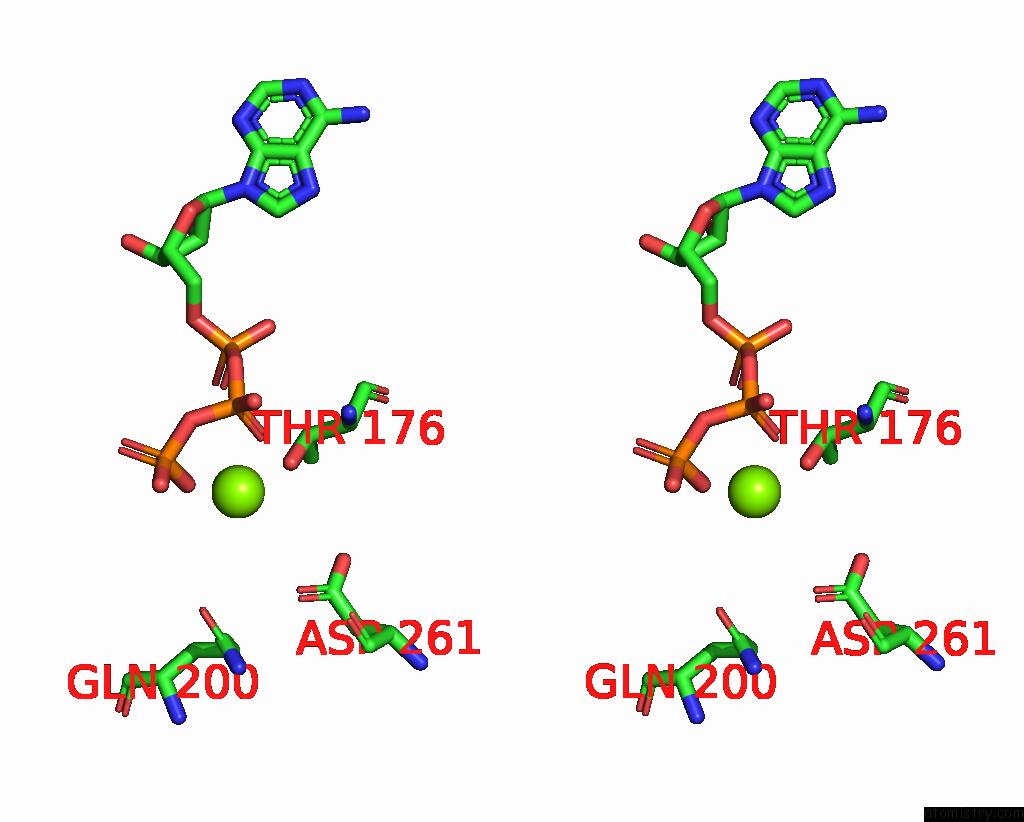
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp within 5.0Å range:
|
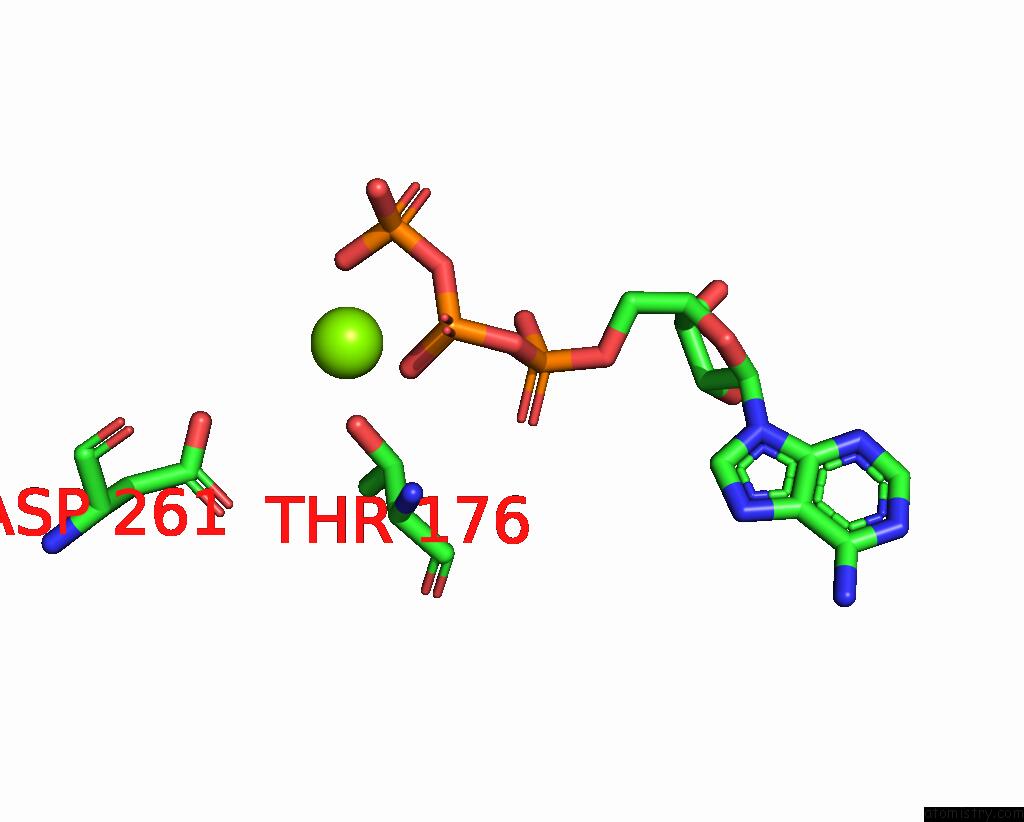
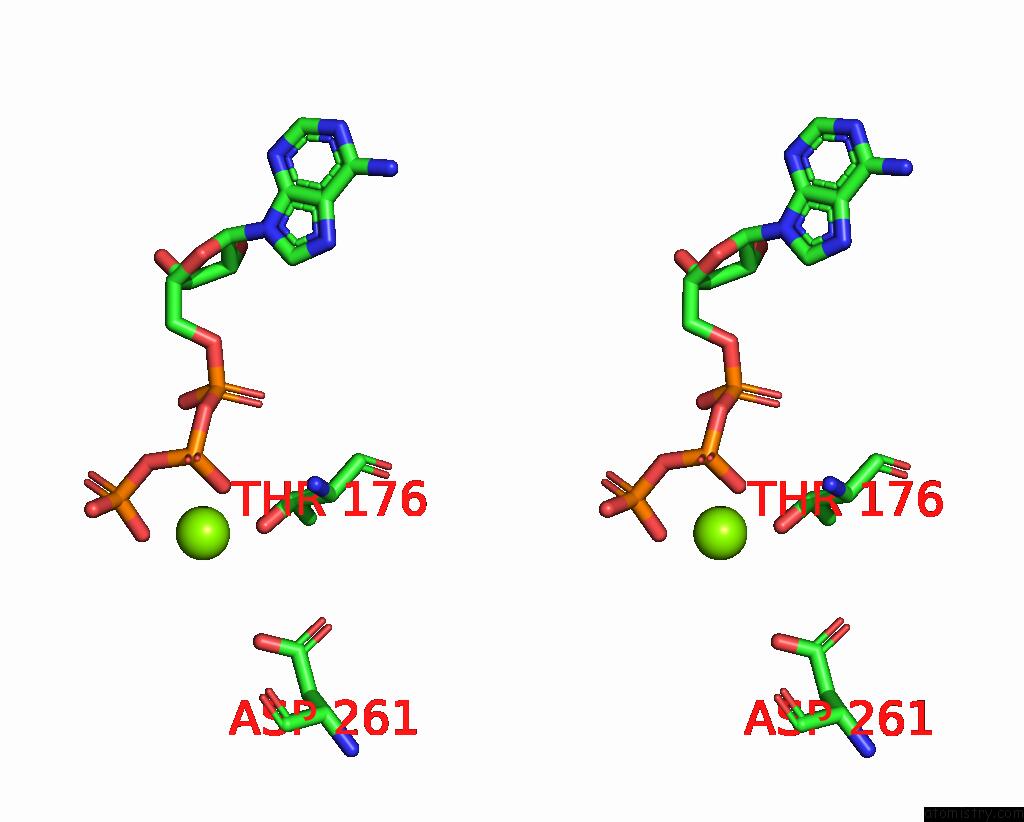
Magnesium binding site 3 out of 5 in 8hh1
Go back to
Magnesium binding site 3 out
of 5 in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp within 5.0Å range:
|
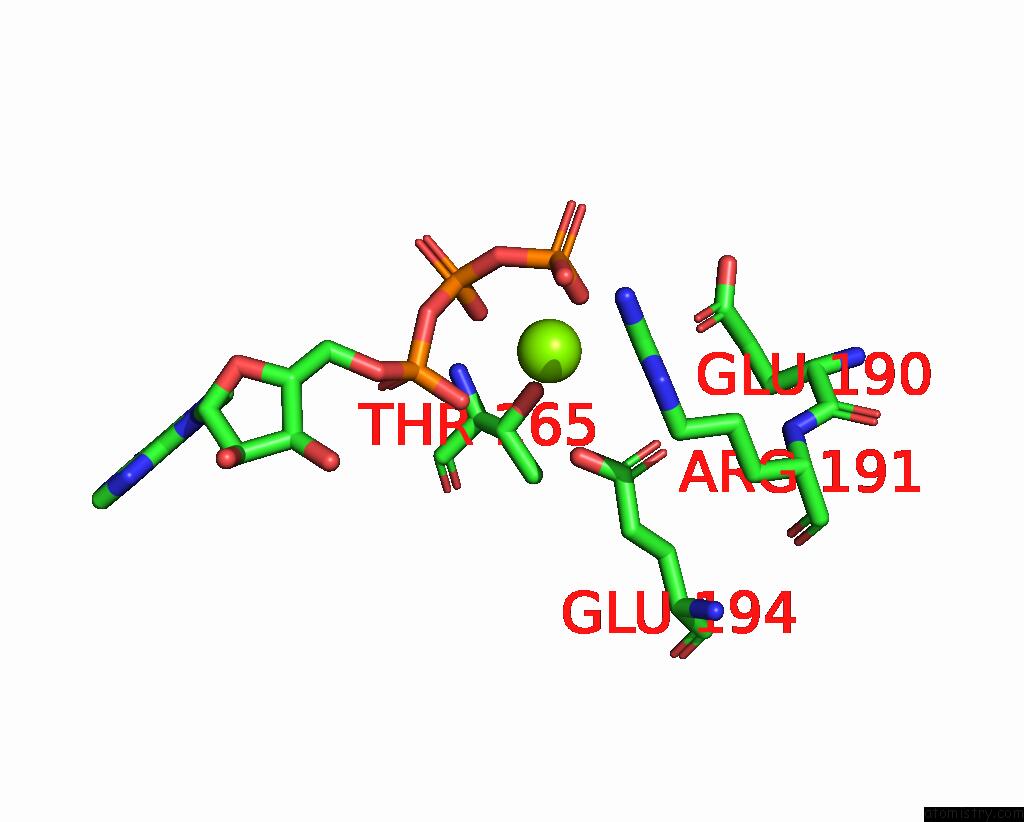
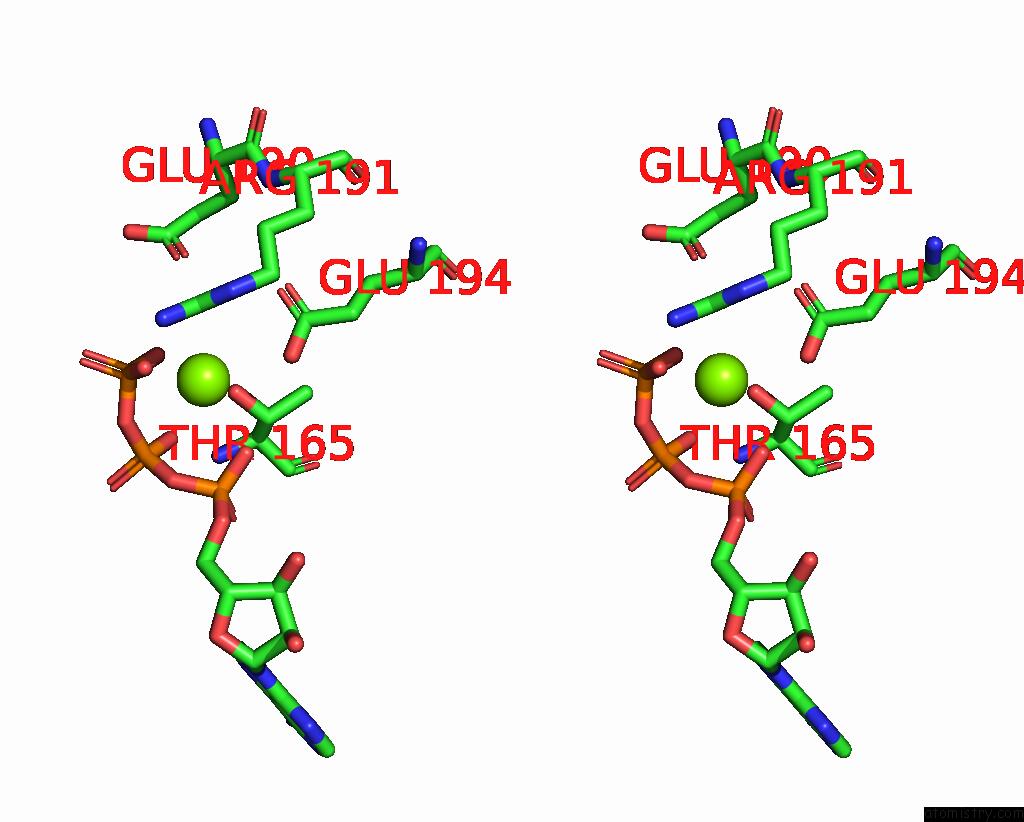
Magnesium binding site 4 out of 5 in 8hh1
Go back to
Magnesium binding site 4 out
of 5 in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp within 5.0Å range:
|
Magnesium binding site 5 out of 5 in 8hh1
Go back to
Magnesium binding site 5 out
of 5 in the FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp
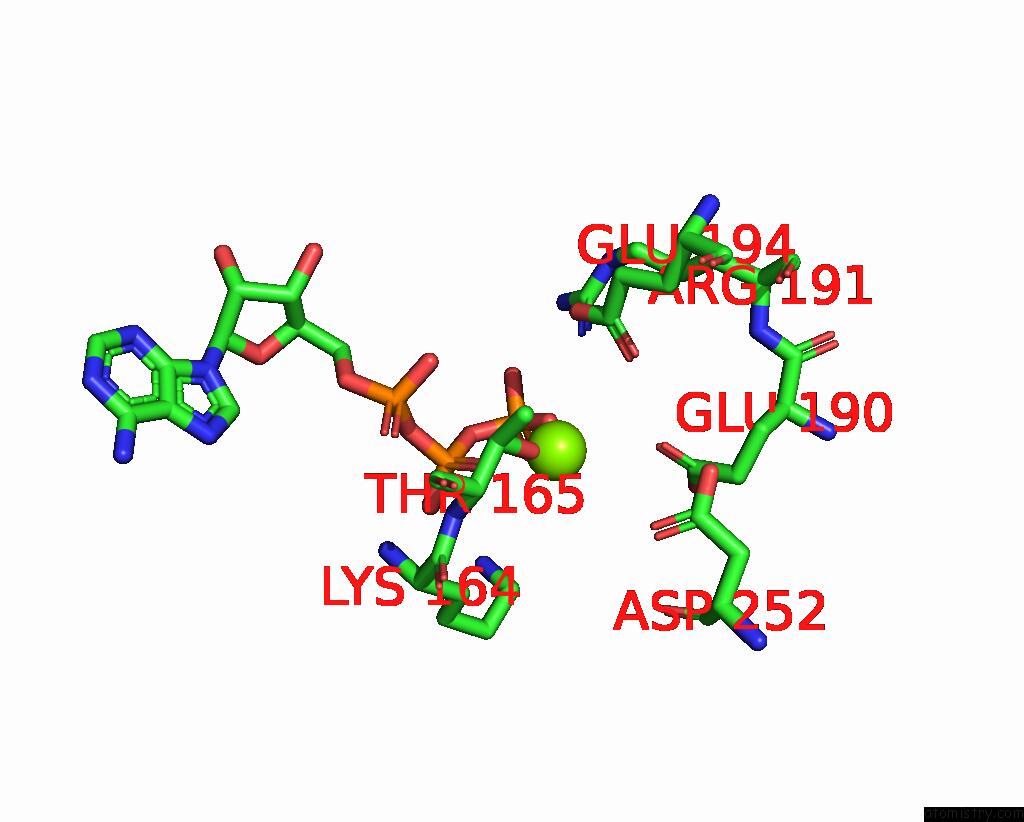
Mono view
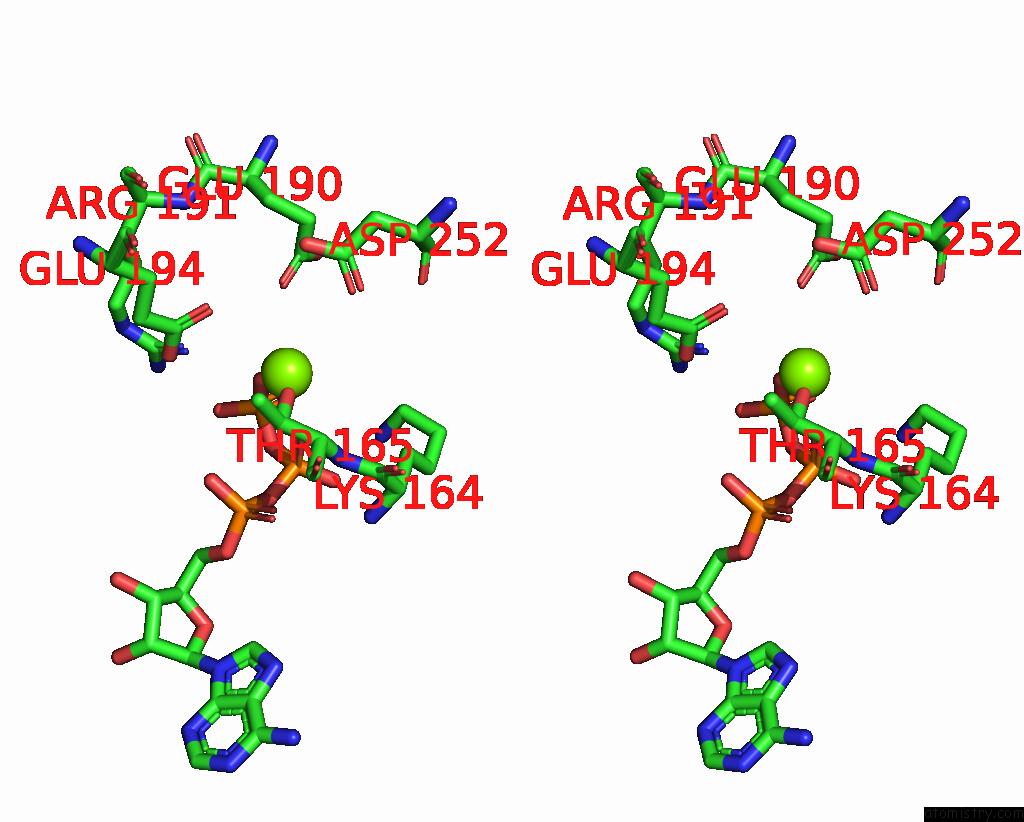
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of FOF1-Atpase From Bacillus PS3, 81 Degrees, Highatp within 5.0Å range:
|
Reference:
A.Nakano,
K.Yokoyama,
J.Kishikawa,
K.Mitsuoka.
Rotation Mechanism of Atp Synthases Driven By Atp Hydrolysis To Be Published.
Page generated: Fri Oct 4 04:52:05 2024
Last articles
F in 7KBGF in 7KBN
F in 7KBC
F in 7KAF
F in 7KA1
F in 7K77
F in 7K8G
F in 7K89
F in 7K87
F in 7K6Z