Magnesium »
PDB 8ijl-8iri »
8io8 »
Magnesium in PDB 8io8: Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly
(pdb code 8io8). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly, PDB code: 8io8:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly, PDB code: 8io8:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8io8
Go back to
Magnesium binding site 1 out
of 2 in the Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly
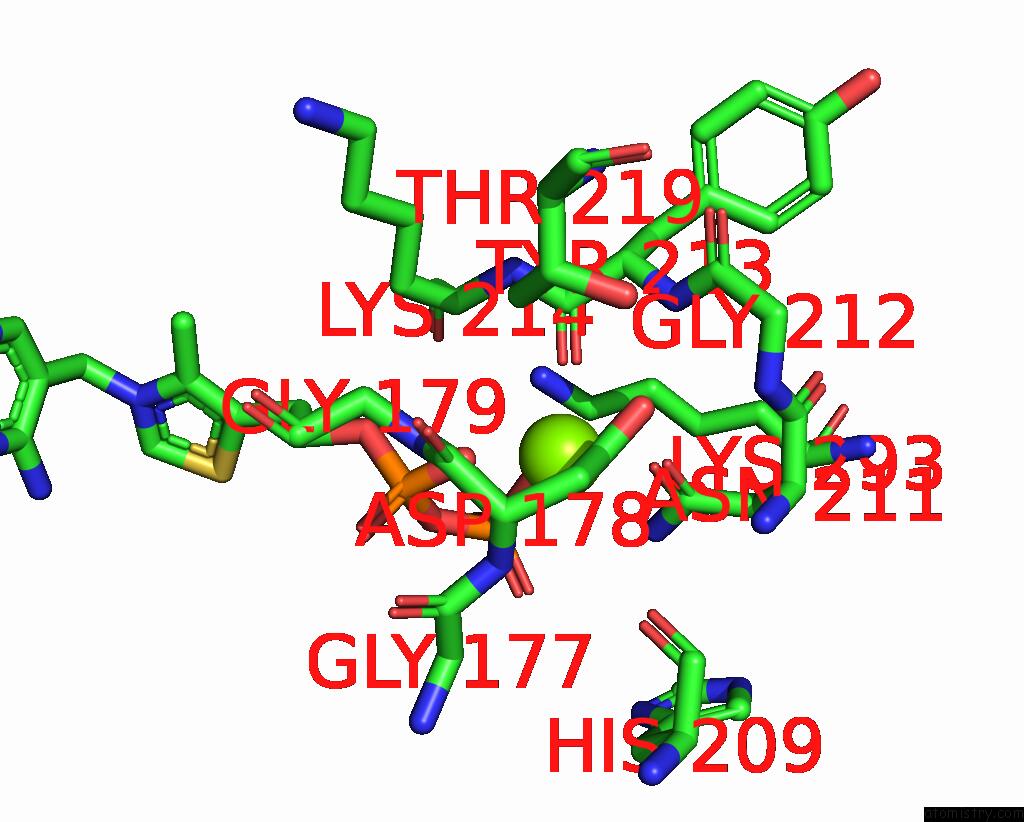
Mono view
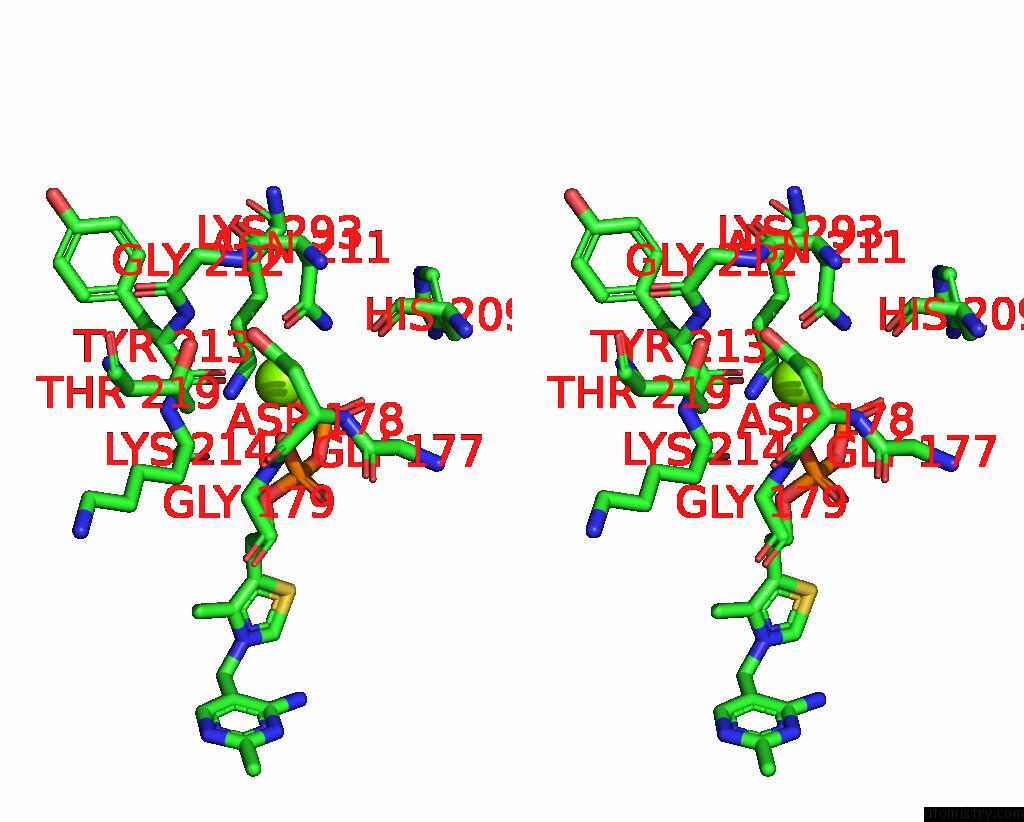
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8io8
Go back to
Magnesium binding site 2 out
of 2 in the Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly
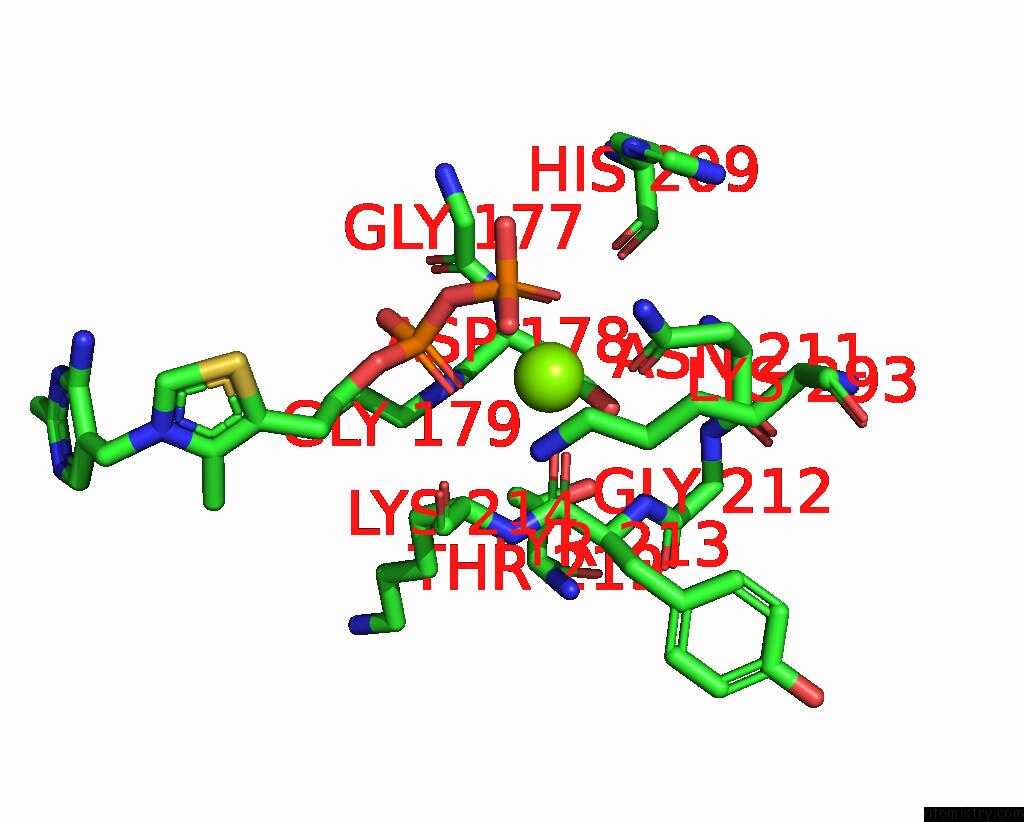
Mono view
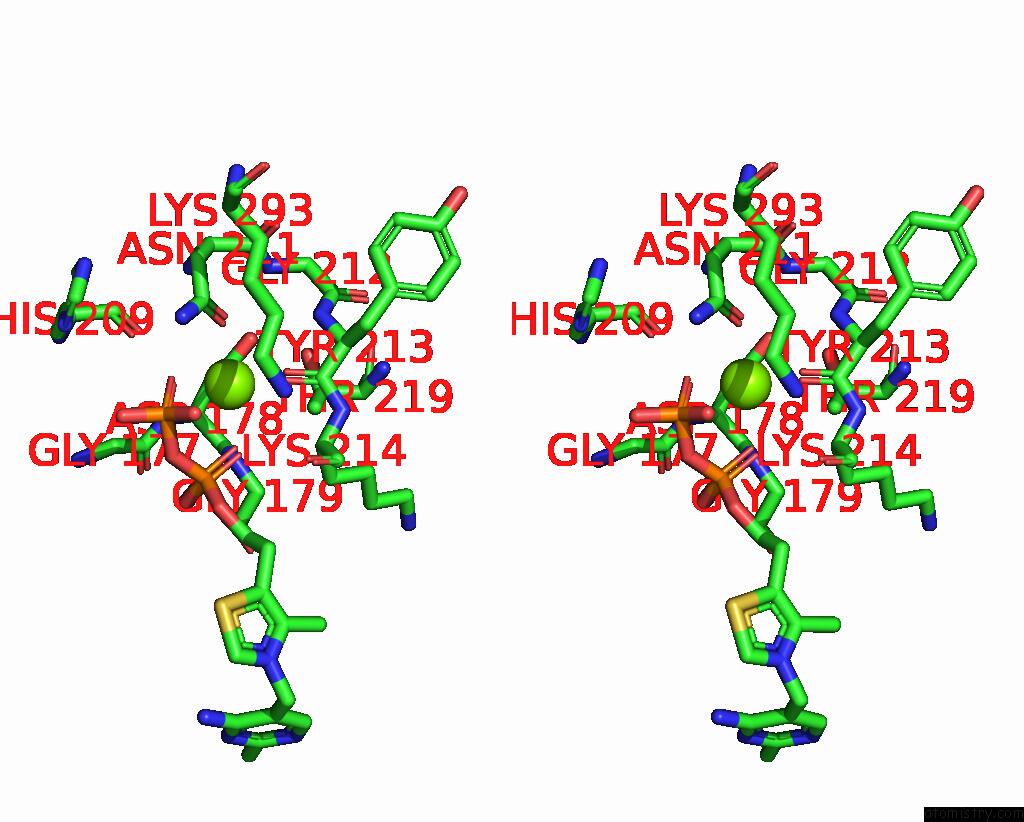
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Cryo-Em Structure of Cyanobacteria Phosphoketolase Complexed with Amppnpin Dimeric Assembly within 5.0Å range:
|
Reference:
C.-W.Chang,
M.-D.Tsai.
An Atp-Sensitive Phosphoketolase Regulates Carbon Fixation in Cyanobacteria. Nat Metab 2023.
ISSN: ISSN 2522-5812
DOI: 10.1038/S42255-023-00831-W
Page generated: Fri Oct 4 09:29:44 2024
ISSN: ISSN 2522-5812
DOI: 10.1038/S42255-023-00831-W
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF