Magnesium »
PDB 8p5v-8pj9 »
8pfj »
Magnesium in PDB 8pfj: Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah)
Enzymatic activity of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah)
All present enzymatic activity of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah):
2.7.7.6;
2.7.7.6;
Other elements in 8pfj:
The structure of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah) also contains other interesting chemical elements:
| Zinc | (Zn) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah)
(pdb code 8pfj). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah), PDB code: 8pfj:
In total only one binding site of Magnesium was determined in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah), PDB code: 8pfj:
Magnesium binding site 1 out of 1 in 8pfj
Go back to
Magnesium binding site 1 out
of 1 in the Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah)
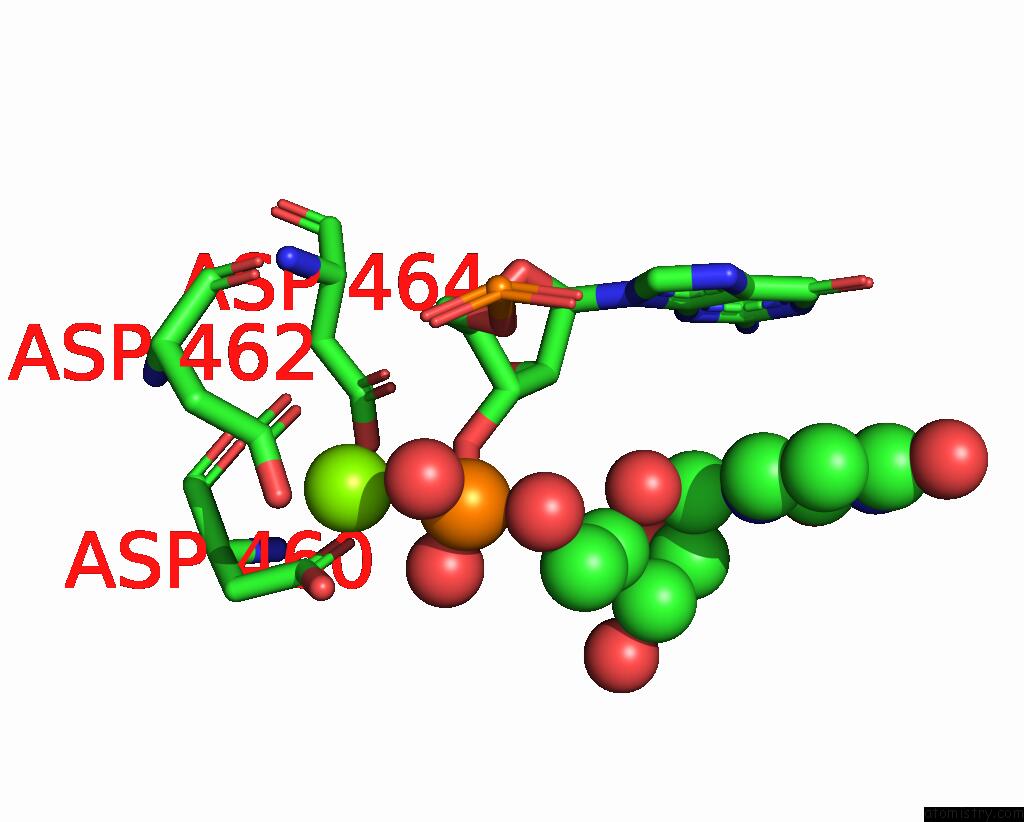
Mono view
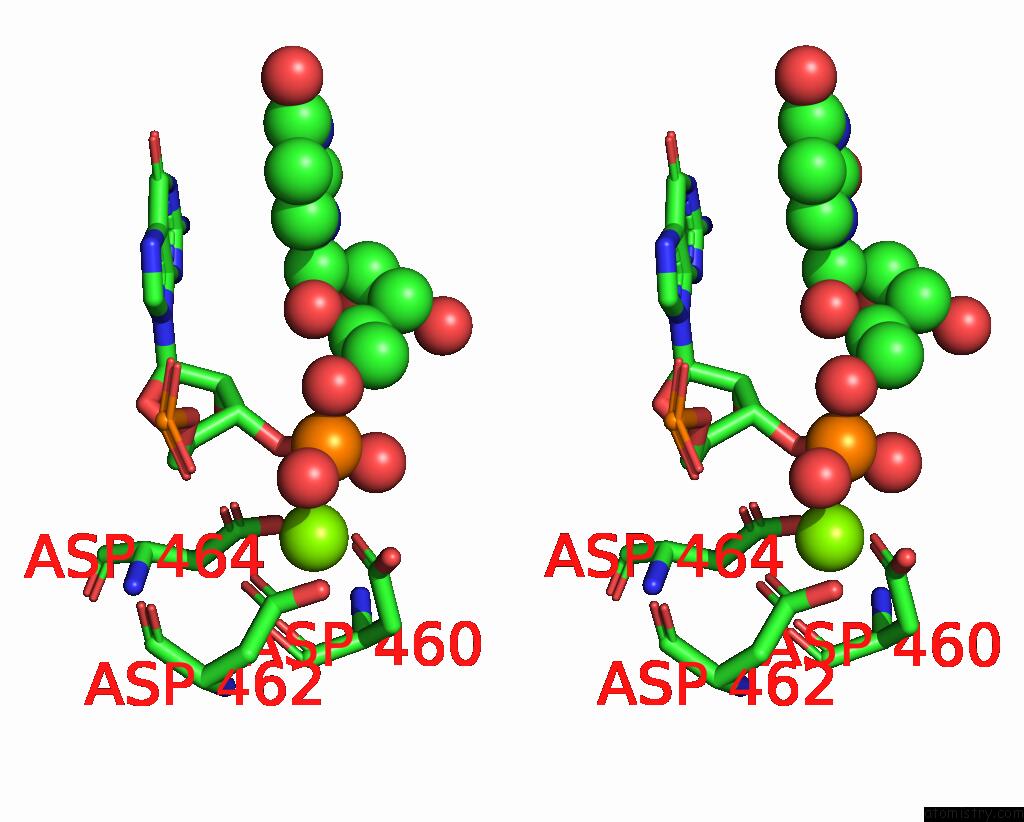
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Fully Recruited Rfah Bound to E. Coli Transcription Complex Paused at Ops Site (Not Fully Complementary Scaffold; Alternative State of Rfah) within 5.0Å range:
|
Reference:
P.K.Zuber,
N.Said,
T.Hilal,
B.Wang,
B.Loll,
J.Gonzalez-Higueras,
C.A.Ramirez-Sarmiento,
G.A.Belogurov,
I.Artsimovitch,
M.C.Wahl,
S.H.Knauer.
Concerted Transformation of A Hyper-Paused Transcription Complex and Its Reinforcing Protein. Nat Commun V. 15 3040 2024.
ISSN: ESSN 2041-1723
PubMed: 38589445
DOI: 10.1038/S41467-024-47368-4
Page generated: Fri Oct 4 16:03:03 2024
ISSN: ESSN 2041-1723
PubMed: 38589445
DOI: 10.1038/S41467-024-47368-4
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF