Magnesium »
PDB 8vbi-8vn3 »
8vh5 »
Magnesium in PDB 8vh5: Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State
Enzymatic activity of Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State
All present enzymatic activity of Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State:
2.7.11.1;
2.7.11.1;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State
(pdb code 8vh5). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State, PDB code: 8vh5:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State, PDB code: 8vh5:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8vh5
Go back to
Magnesium binding site 1 out
of 2 in the Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State
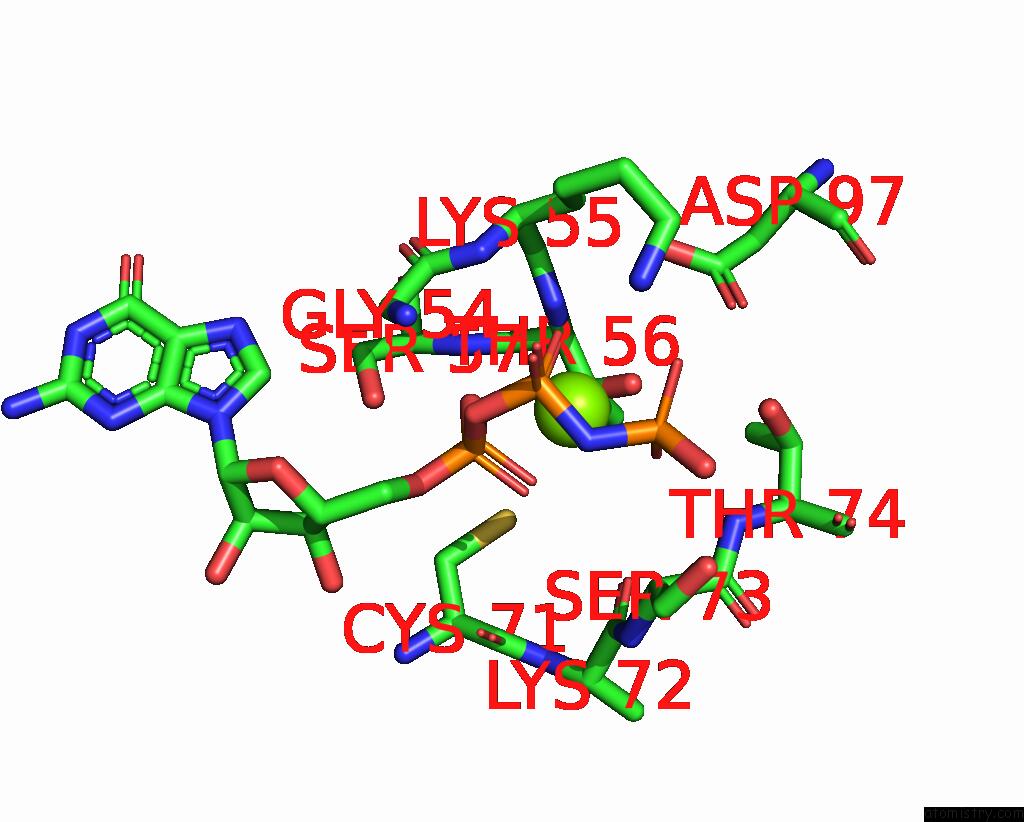
Mono view
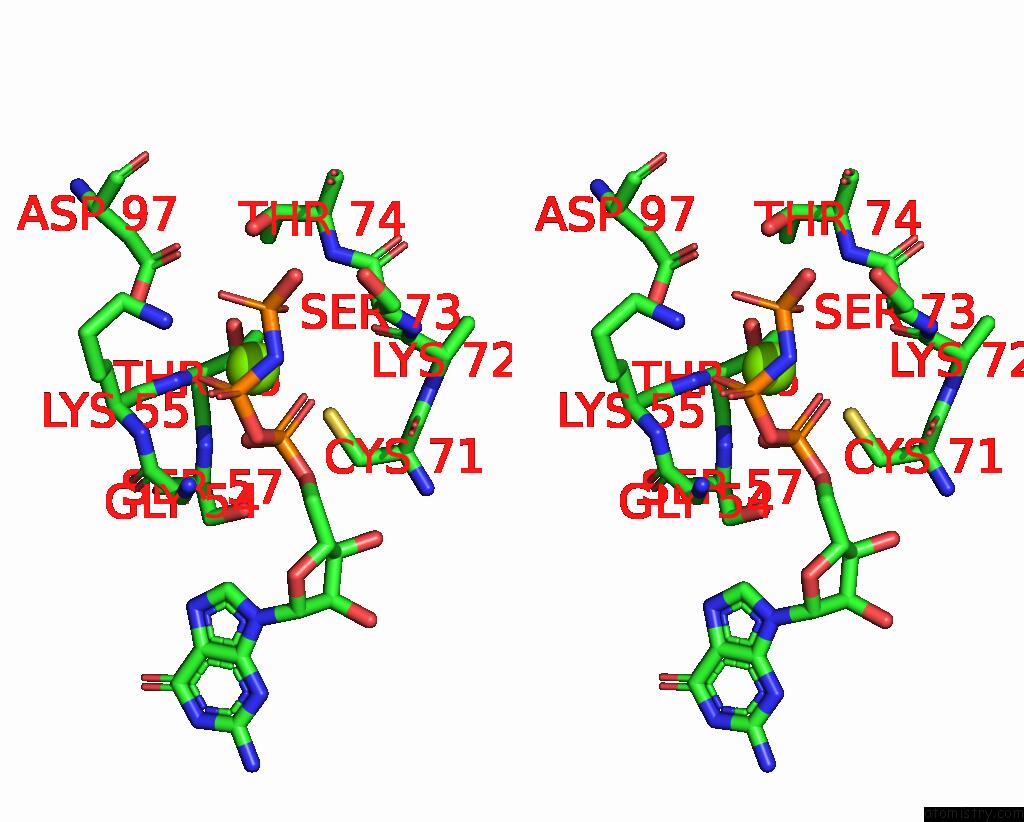
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8vh5
Go back to
Magnesium binding site 2 out
of 2 in the Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State
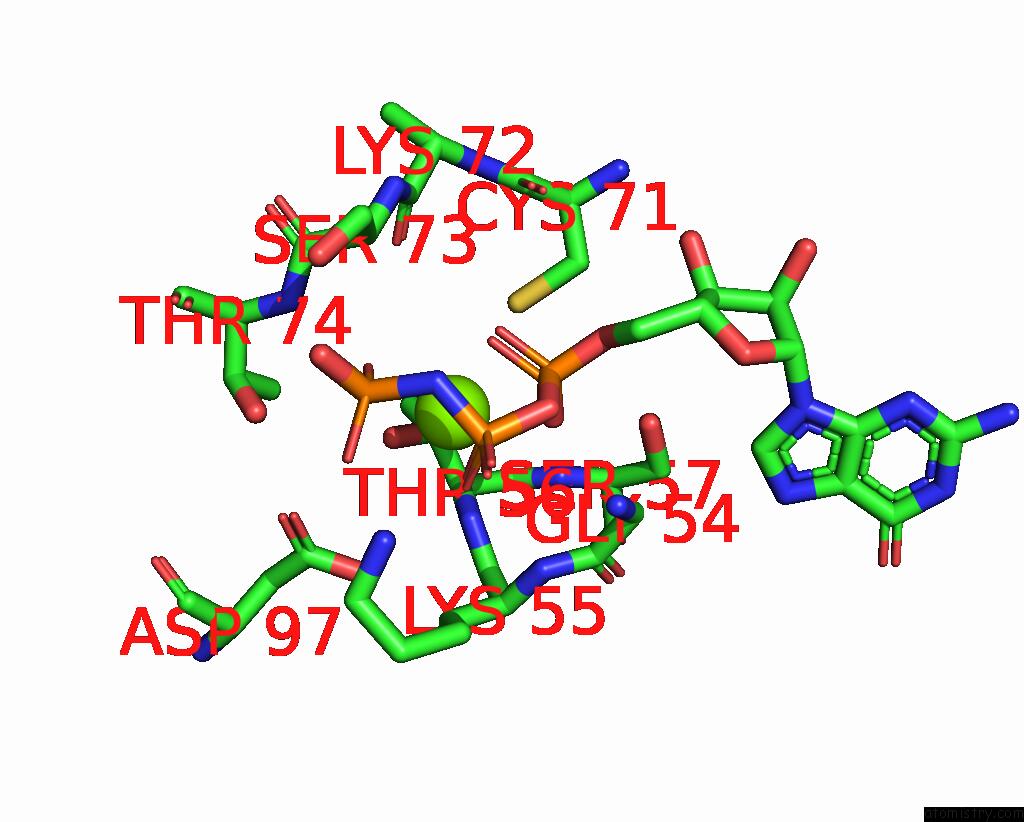
Mono view
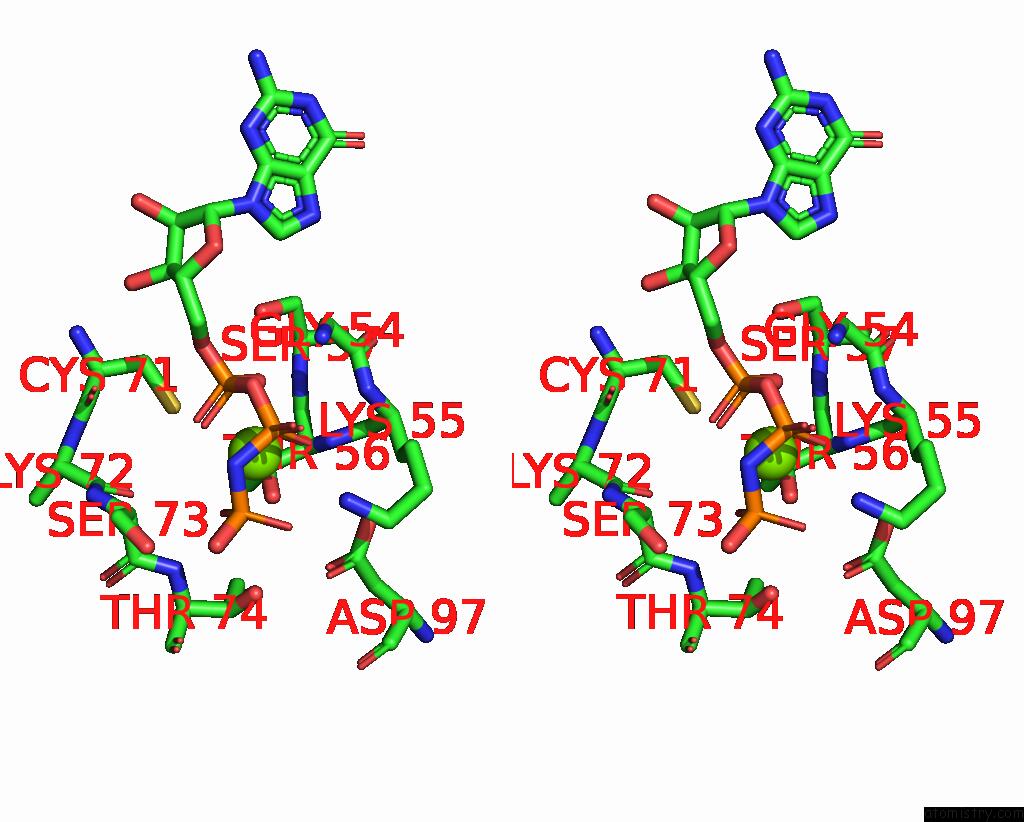
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Cryo-Em Structure of RAB12-LRRK2 Complex in the LRRK2 Dimer State within 5.0Å range:
|
Reference:
X.Li,
H.Zhu,
B.T.Huang,
X.Li,
H.Kim,
H.Tan,
Y.Zhang,
I.Choi,
J.Peng,
P.Xu,
J.Sun,
Z.Yue.
RAB12-LRRK2 Complex Suppresses Primary Ciliogenesis and Regulates Centrosome Homeostasis in Astrocytes. Nat Commun V. 15 8434 2024.
ISSN: ESSN 2041-1723
PubMed: 39343966
DOI: 10.1038/S41467-024-52723-6
Page generated: Fri Aug 15 17:37:38 2025
ISSN: ESSN 2041-1723
PubMed: 39343966
DOI: 10.1038/S41467-024-52723-6
Last articles
Mg in 8WGHMg in 8WMO
Mg in 8WMN
Mg in 8WMM
Mg in 8WEY
Mg in 8WKG
Mg in 8WKF
Mg in 8WIM
Mg in 8WIL
Mg in 8WH2