Magnesium »
PDB 1c5u-1cxz »
1cjk »
Magnesium in PDB 1cjk: Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn
Enzymatic activity of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn
All present enzymatic activity of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn:
4.6.1.1;
4.6.1.1;
Protein crystallography data
The structure of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn, PDB code: 1cjk
was solved by
J.J.G.Tesmer,
S.R.Sprang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 15.00 / 3.00 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 118.900, 134.900, 72.200, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22 / 26.6 |
Other elements in 1cjk:
The structure of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn also contains other interesting chemical elements:
| Manganese | (Mn) | 1 atom |
| Chlorine | (Cl) | 1 atom |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn
(pdb code 1cjk). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn, PDB code: 1cjk:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn, PDB code: 1cjk:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1cjk
Go back to
Magnesium binding site 1 out
of 2 in the Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn
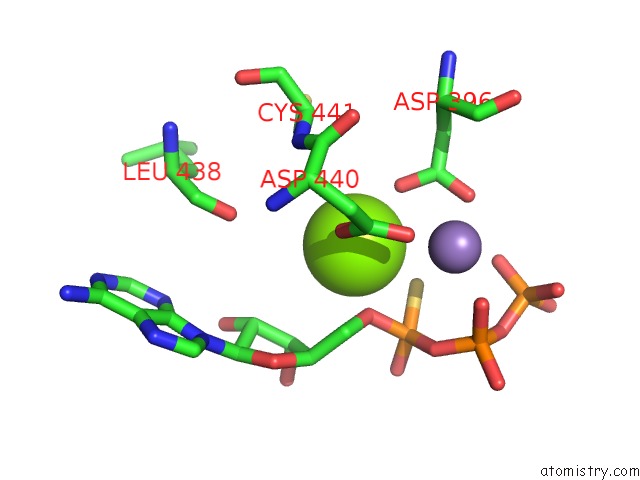
Mono view
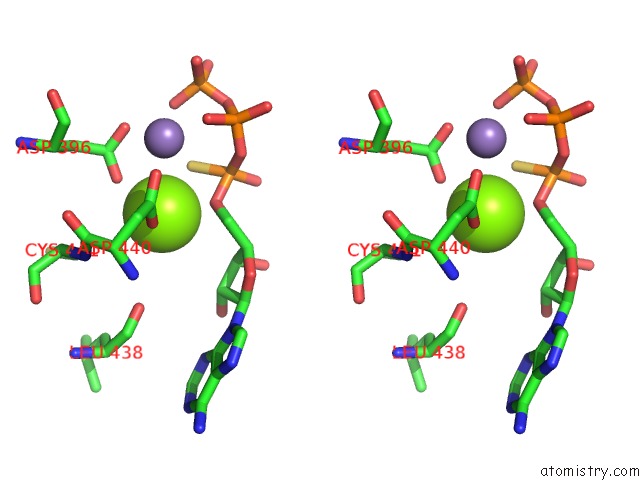
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1cjk
Go back to
Magnesium binding site 2 out
of 2 in the Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn
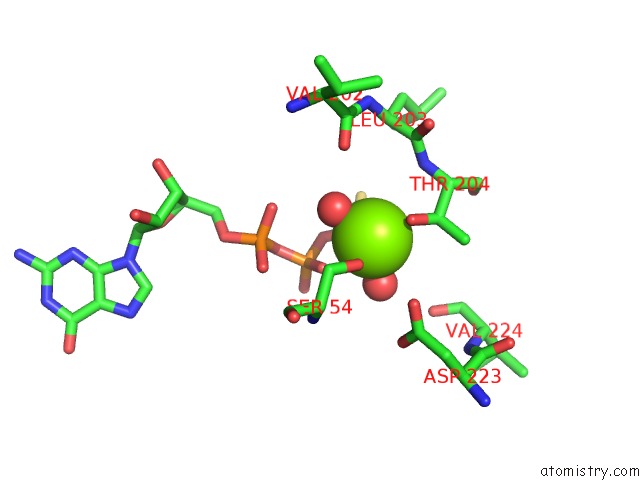
Mono view
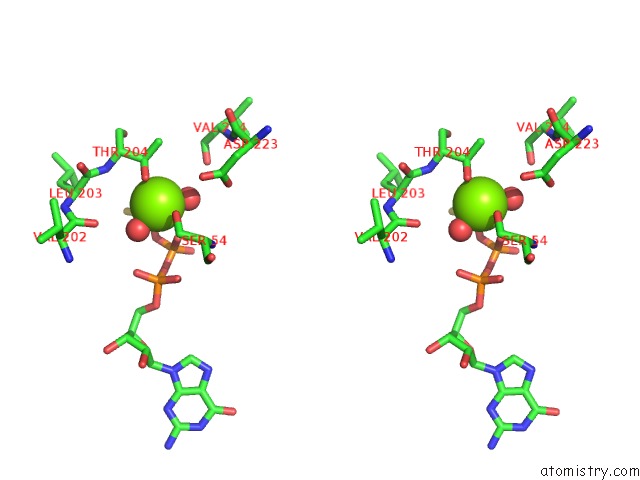
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Complex of Gs-Alpha with the Catalytic Domains of Mammalian Adenylyl Cyclase: Complex with Adenosine 5'-(Alpha Thio)-Triphosphate (Rp), Mg, and Mn within 5.0Å range:
|
Reference:
J.J.Tesmer,
R.K.Sunahara,
R.A.Johnson,
G.Gosselin,
A.G.Gilman,
S.R.Sprang.
Two-Metal-Ion Catalysis in Adenylyl Cyclase. Science V. 285 756 1999.
ISSN: ISSN 0036-8075
PubMed: 10427002
DOI: 10.1126/SCIENCE.285.5428.756
Page generated: Tue Aug 13 02:29:05 2024
ISSN: ISSN 0036-8075
PubMed: 10427002
DOI: 10.1126/SCIENCE.285.5428.756
Last articles
K in 4LF5K in 4LF4
K in 4LE2
K in 4LCU
K in 4LCA
K in 4LC4
K in 4LBX
K in 4LBG
K in 4LBE
K in 4L6A