Magnesium »
PDB 1x1t-1xfx »
1xdg »
Magnesium in PDB 1xdg: X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution
Protein crystallography data
The structure of X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution, PDB code: 1xdg
was solved by
G.Weitz-Schmidt,
K.Welzenbach,
J.Dawson,
J.Kallen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 8.00 / 2.10 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 71.900, 77.400, 92.300, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.1 / 21.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution
(pdb code 1xdg). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution, PDB code: 1xdg:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution, PDB code: 1xdg:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1xdg
Go back to
Magnesium binding site 1 out
of 2 in the X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution
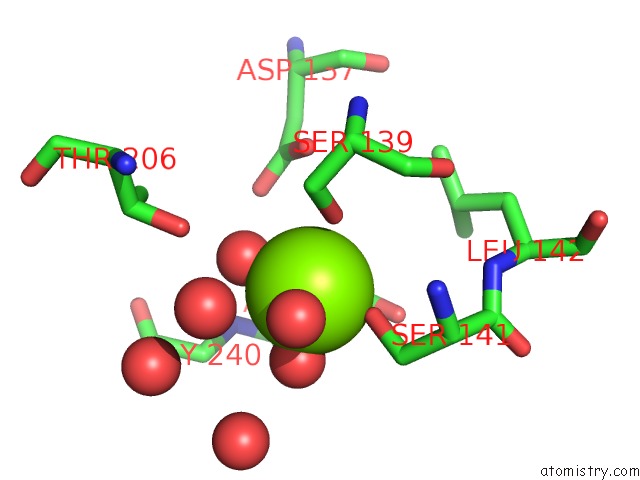
Mono view
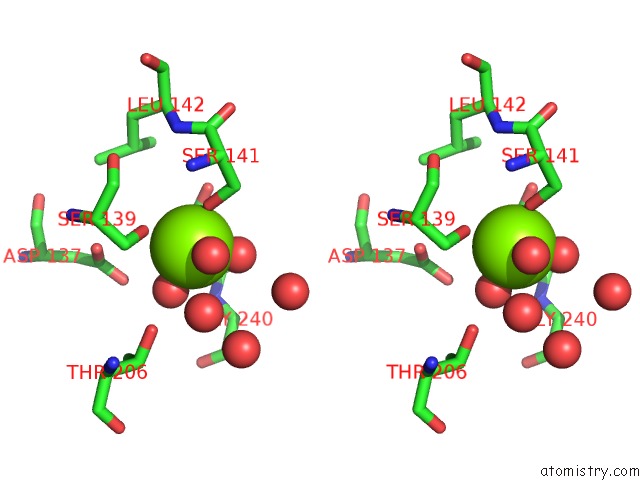
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1xdg
Go back to
Magnesium binding site 2 out
of 2 in the X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution
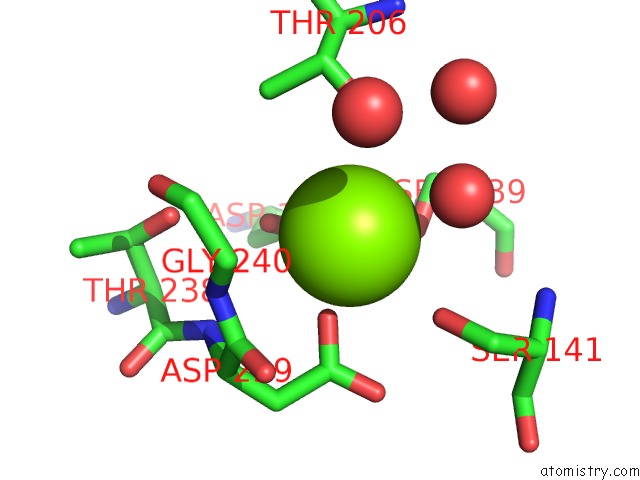
Mono view
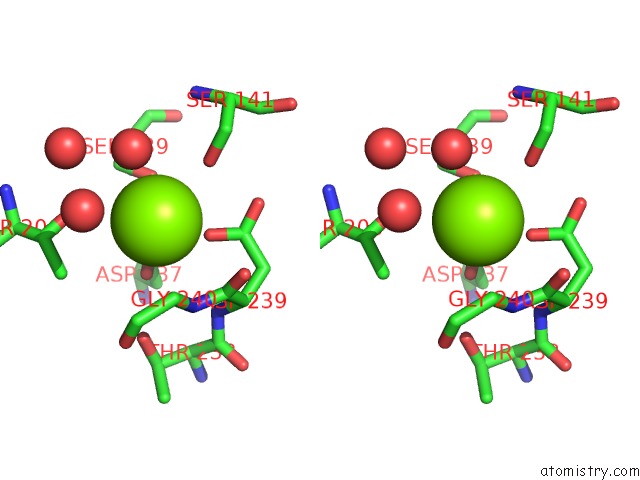
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of X-Ray Structure of Lfa-1 I-Domain in Complex with LFA878 at 2.1A Resolution within 5.0Å range:
|
Reference:
G.Weitz-Schmidt,
K.Welzenbach,
J.Dawson,
J.Kallen.
Improved Lymphocyte Function-Associated Antigen-1 (Lfa-1) Inhibition By Statin Derivatives: Molecular Basis Determined By X-Ray Analysis and Monitoring of Lfa-1 Conformational Changes in Vitro and Ex Vivo J.Biol.Chem. V. 279 46764 2004.
ISSN: ISSN 0021-9258
PubMed: 15304496
DOI: 10.1074/JBC.M407951200
Page generated: Tue Aug 13 17:31:47 2024
ISSN: ISSN 0021-9258
PubMed: 15304496
DOI: 10.1074/JBC.M407951200
Last articles
K in 7FS8K in 7FS6
K in 7FS5
K in 7FS7
K in 7FS1
K in 7FS3
K in 7FS4
K in 7FS2
K in 7FS0
K in 7FRZ