Magnesium »
PDB 3jxq-3k9f »
3k8d »
Magnesium in PDB 3k8d: Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
Enzymatic activity of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
All present enzymatic activity of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo:
2.7.7.38;
2.7.7.38;
Protein crystallography data
The structure of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo, PDB code: 3k8d
was solved by
D.J.Heyes,
C.W.Levy,
P.Lafite,
N.S.Scrutton,
D.Leys,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.27 / 1.90 |
| Space group | P 31 |
| Cell size a, b, c (Å), α, β, γ (°) | 94.770, 94.770, 153.140, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 15.1 / 18.2 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
(pdb code 3k8d). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo, PDB code: 3k8d:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo, PDB code: 3k8d:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 3k8d
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
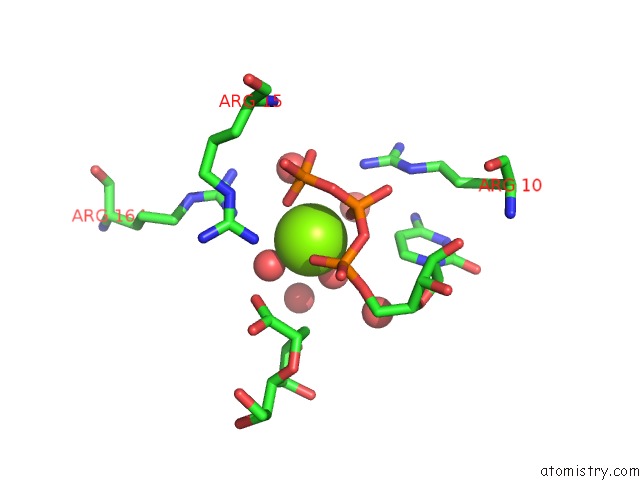
Mono view
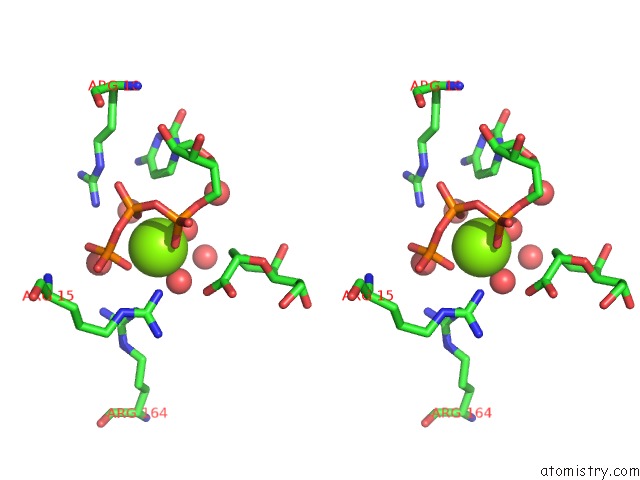
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 3k8d
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
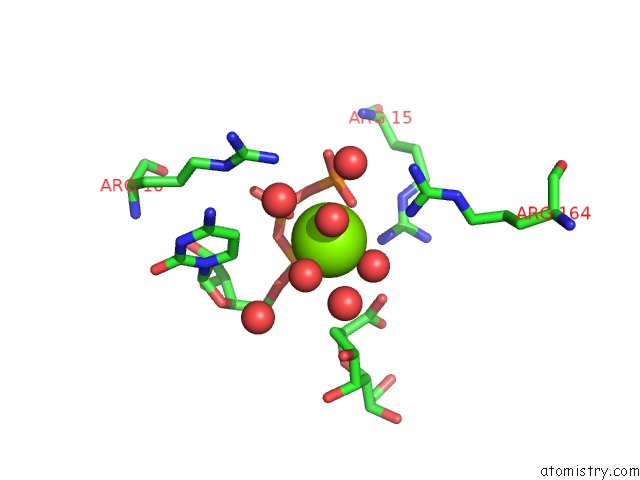
Mono view
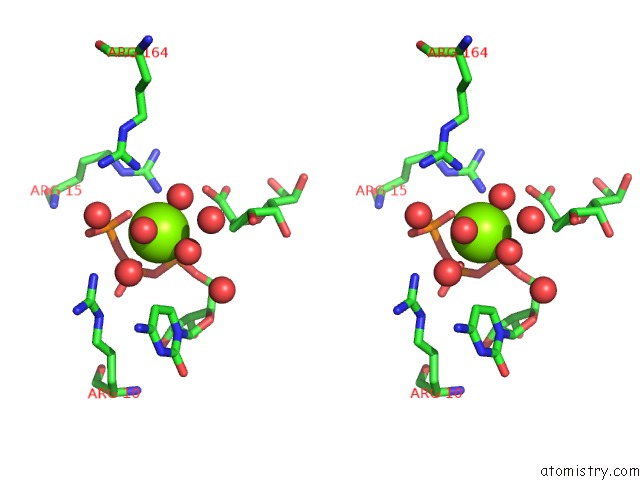
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 3k8d
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
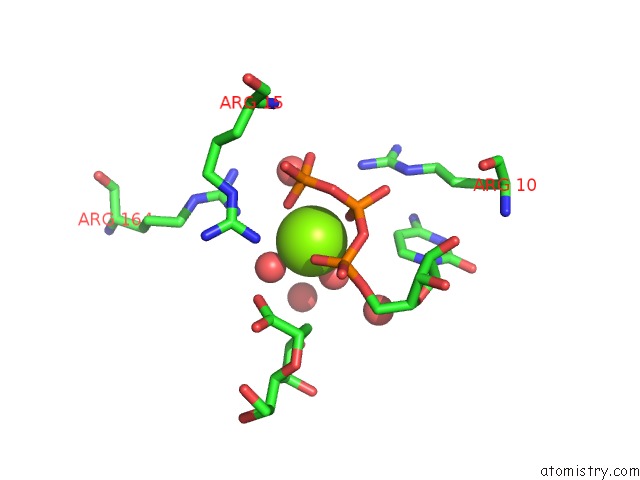
Mono view
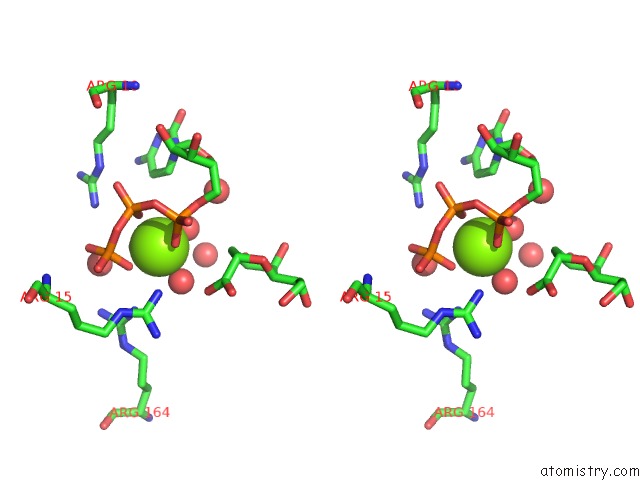
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 3k8d
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo
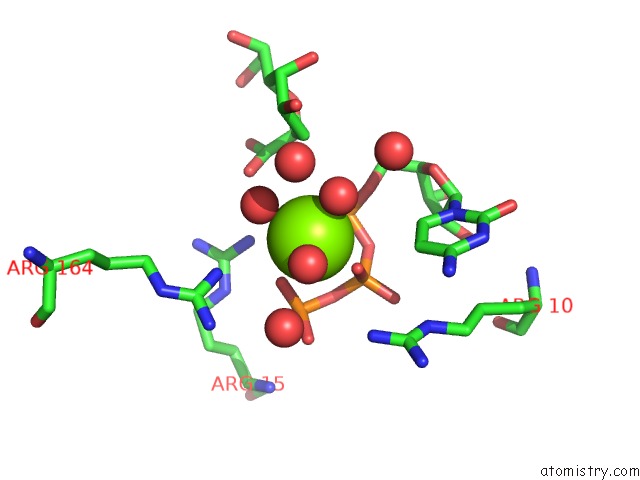
Mono view
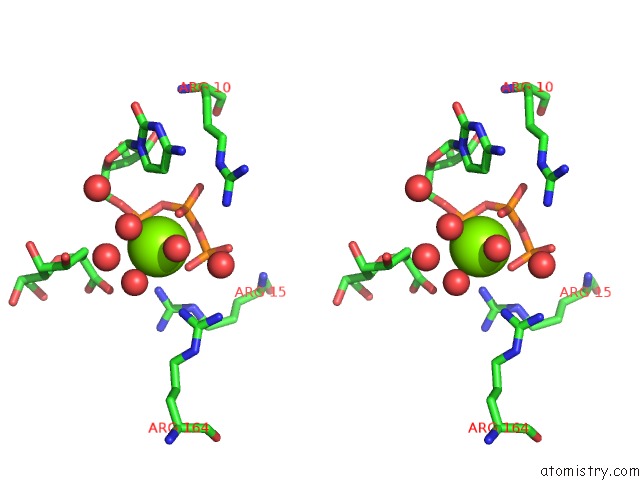
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of E. Coli Lipopolysaccharide Specific Cmp-Kdo Synthetase in Complex with Ctp and 2-Deoxy-Kdo within 5.0Å range:
|
Reference:
D.J.Heyes,
C.Levy,
P.Lafite,
I.S.Roberts,
M.Goldrick,
A.V.Stachulski,
S.B.Rossington,
D.Stanford,
S.E.J.Rigby,
N.S.Scrutton,
D.Leys.
Structure-Based Mechanism of Cmp-2-Keto-3-Deoxymanno-Octulonic Acid Synthetase: Convergent Evolution of A Sugar-Activating Enzyme with Dna/Rna Polymerases J.Biol.Chem. V. 284 35514 2009.
ISSN: ISSN 0021-9258
PubMed: 19815542
DOI: 10.1074/JBC.M109.056630
Page generated: Wed Aug 14 17:57:02 2024
ISSN: ISSN 0021-9258
PubMed: 19815542
DOI: 10.1074/JBC.M109.056630
Last articles
K in 1S5HK in 1S61
K in 1RXC
K in 1S56
K in 1RY5
K in 1RXY
K in 1RXI
K in 1RXE
K in 1RG9
K in 1RRV