Magnesium »
PDB 4ko8-4kvw »
4kr5 »
Magnesium in PDB 4kr5: Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation
Protein crystallography data
The structure of Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation, PDB code: 4kr5
was solved by
A.Vujicic Zagar,
A.Guskov,
G.K.Schuurman-Wolters,
D.J.Slotboom,
B.Poolman,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.89 / 1.50 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.691, 89.123, 59.477, 90.00, 95.58, 90.00 |
| R / Rfree (%) | 14.3 / 18.6 |
Other elements in 4kr5:
The structure of Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation also contains other interesting chemical elements:
| Chlorine | (Cl) | 4 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation
(pdb code 4kr5). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation, PDB code: 4kr5:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation, PDB code: 4kr5:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4kr5
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation
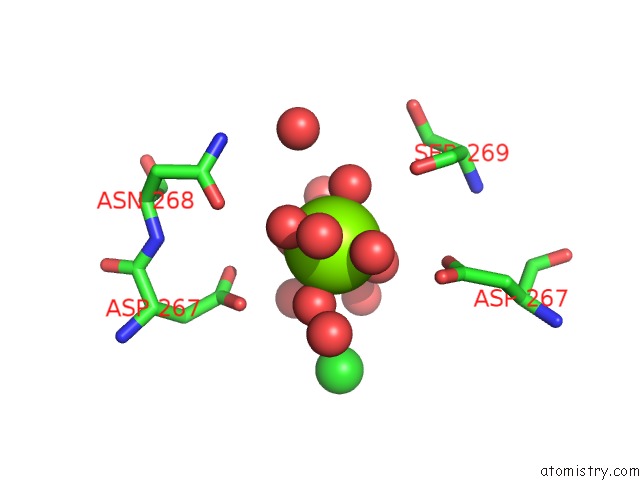
Mono view
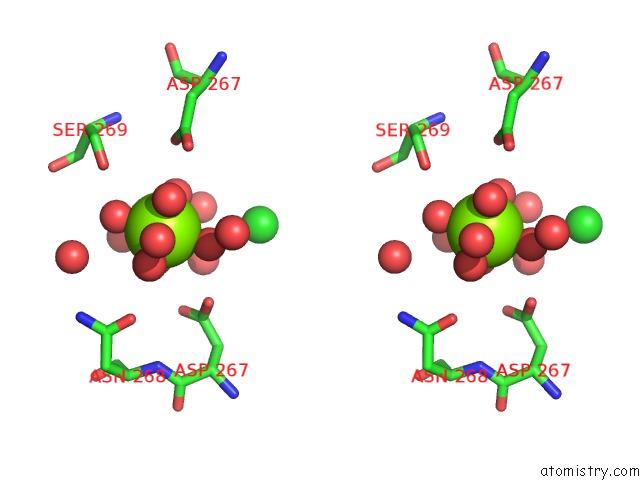
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4kr5
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation
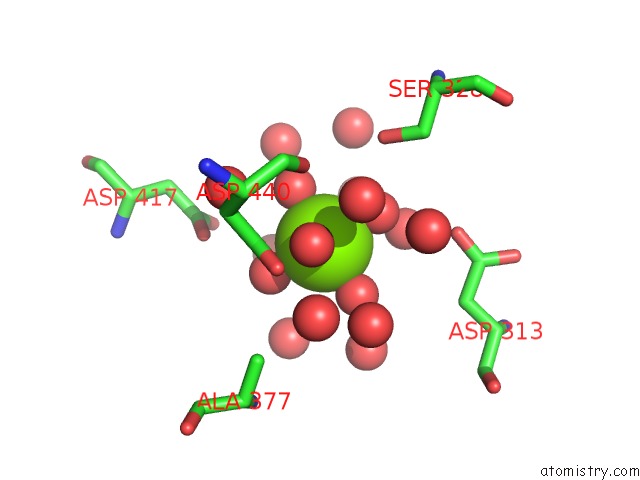
Mono view
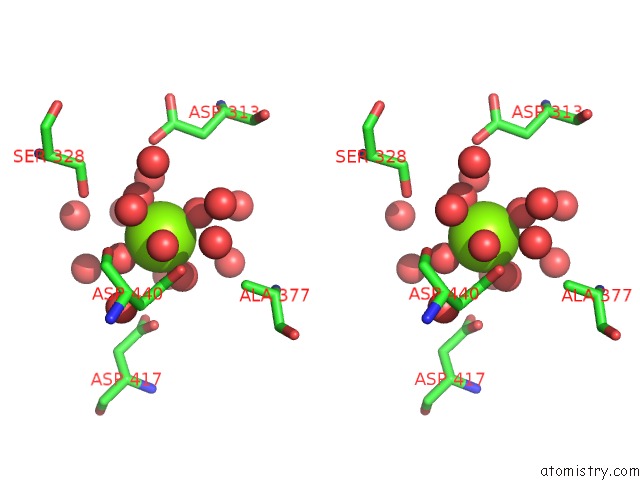
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Lactococcus Lactis Glnp Substrate Binding Domain 2 (SBD2) in Open Conformation within 5.0Å range:
|
Reference:
F.Fulyani,
G.K.Schuurman-Wolters,
A.V.Zagar,
A.Guskov,
D.J.Slotboom,
B.Poolman.
Functional Diversity of Tandem Substrate-Binding Domains in Abc Transporters From Pathogenic Bacteria. Structure V. 21 1879 2013.
ISSN: ISSN 0969-2126
PubMed: 23994008
DOI: 10.1016/J.STR.2013.07.020
Page generated: Mon Aug 11 17:49:16 2025
ISSN: ISSN 0969-2126
PubMed: 23994008
DOI: 10.1016/J.STR.2013.07.020
Last articles
Mg in 7KXGMg in 7KZ2
Mg in 7KZ4
Mg in 7KYB
Mg in 7KYC
Mg in 7KYA
Mg in 7KXH
Mg in 7KY9
Mg in 7KY8
Mg in 7KY7