Magnesium »
PDB 5lmo-5lu4 »
5lpn »
Magnesium in PDB 5lpn: Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1
Protein crystallography data
The structure of Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1, PDB code: 5lpn
was solved by
A.Rai,
A.Oprisko,
J.Campos,
Y.Fu,
T.Friese,
A.Itzen,
R.S.Goody,
M.P.Mueller,
E.M.Gazdag,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.00 / 2.80 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.390, 59.000, 198.180, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 24.4 / 28.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1
(pdb code 5lpn). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1, PDB code: 5lpn:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1, PDB code: 5lpn:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 5lpn
Go back to
Magnesium binding site 1 out
of 2 in the Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1
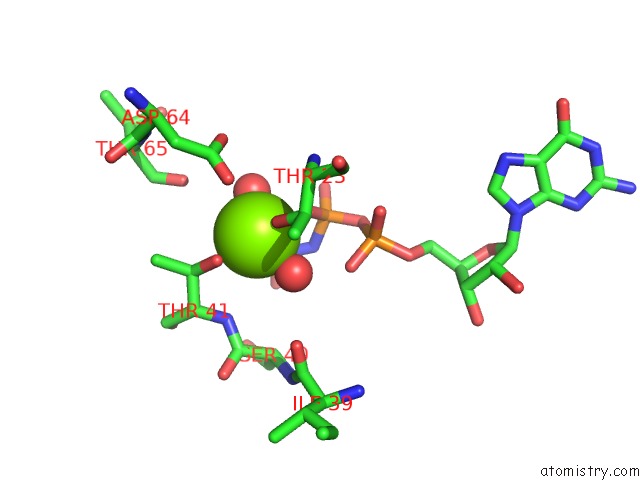
Mono view
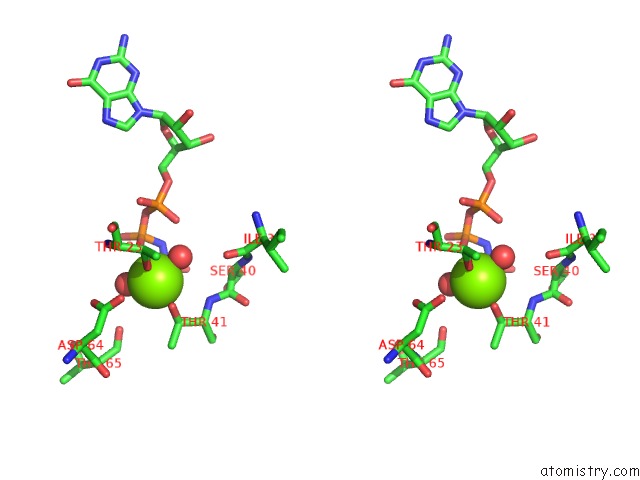
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1 within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 5lpn
Go back to
Magnesium binding site 2 out
of 2 in the Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1
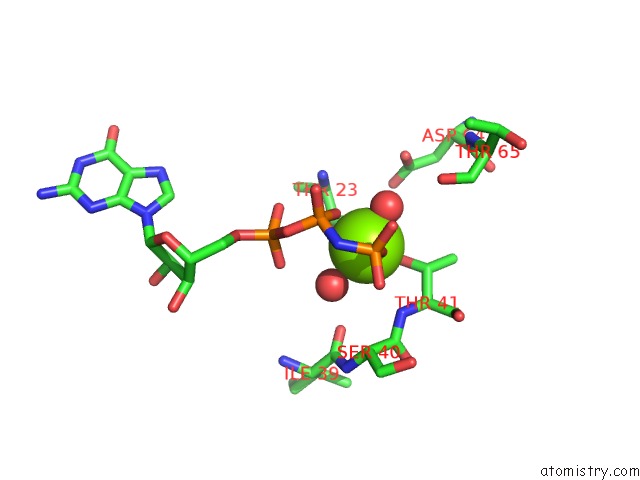
Mono view
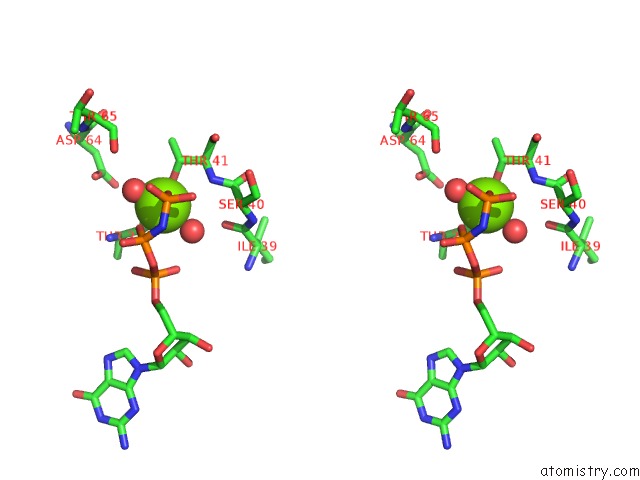
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Human RAB10 in Complex with the Bmerb Domain of Mical-1 within 5.0Å range:
|
Reference:
A.Rai,
A.Oprisko,
J.Campos,
Y.Fu,
T.Friese,
A.Itzen,
R.S.Goody,
E.M.Gazdag,
M.P.Muller.
Bmerb Domains Are Bivalent RAB8 Family Effectors Evolved By Gene Duplication. Elife V. 5 2016.
ISSN: ESSN 2050-084X
PubMed: 27552051
DOI: 10.7554/ELIFE.18675
Page generated: Tue Aug 12 14:38:29 2025
ISSN: ESSN 2050-084X
PubMed: 27552051
DOI: 10.7554/ELIFE.18675
Last articles
Mg in 6CTUMg in 6CTT
Mg in 6CTR
Mg in 6CTQ
Mg in 6CTP
Mg in 6CTO
Mg in 6CTN
Mg in 6CTM
Mg in 6CTL
Mg in 6CT5