Magnesium »
PDB 6r5n-6rdh »
6rcw »
Magnesium in PDB 6rcw: Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
Enzymatic activity of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
All present enzymatic activity of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053:
3.1.4.53;
3.1.4.53;
Protein crystallography data
The structure of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053, PDB code: 6rcw
was solved by
A.K.Singh,
D.G.Brown,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 91.22 / 2.08 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 99.894, 110.505, 160.694, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.2 / 22.1 |
Other elements in 6rcw:
The structure of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053 also contains other interesting chemical elements:
| Zinc | (Zn) | 4 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
(pdb code 6rcw). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053, PDB code: 6rcw:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053, PDB code: 6rcw:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 6rcw
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
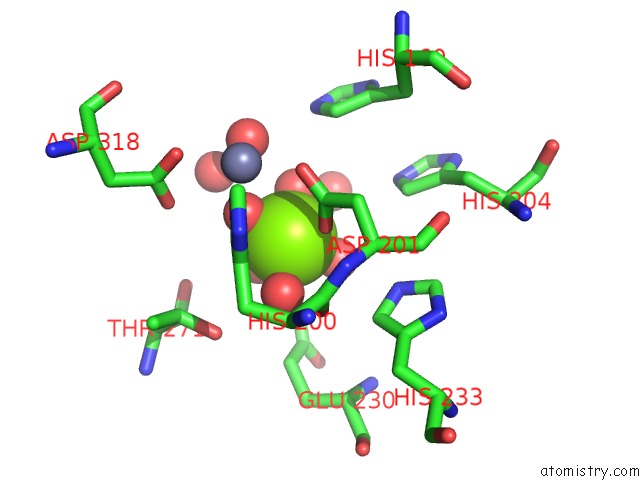
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053 within 5.0Å range:
|
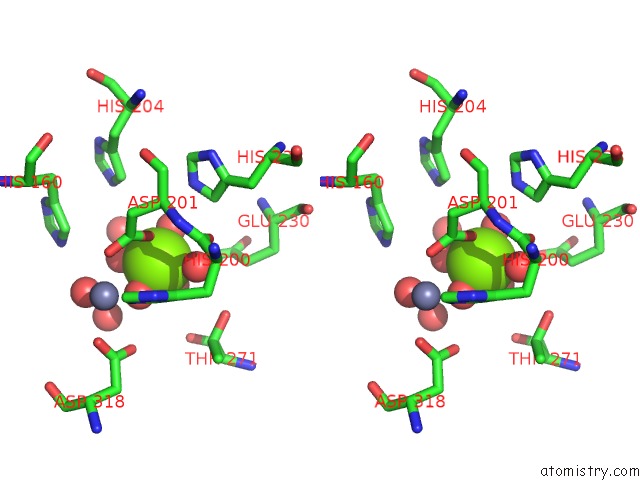
Magnesium binding site 2 out of 4 in 6rcw
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053 within 5.0Å range:
|
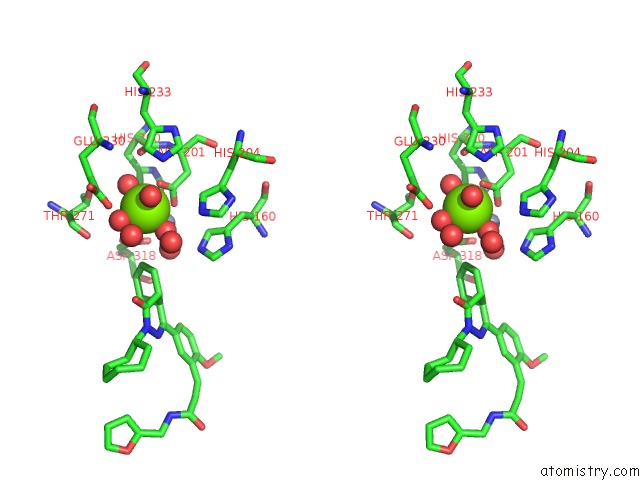
Magnesium binding site 3 out of 4 in 6rcw
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
Mono view
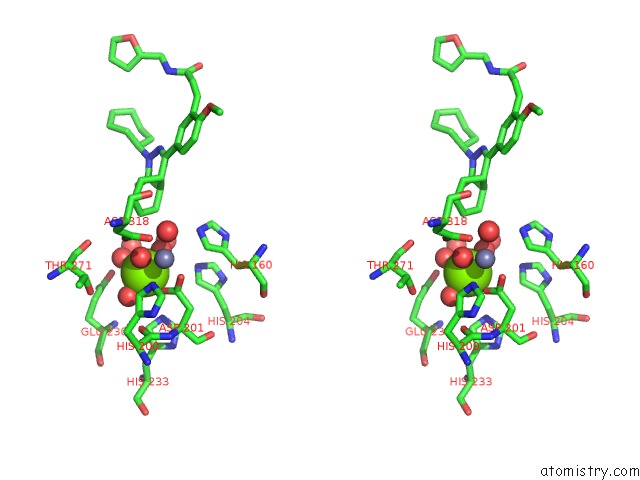
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053 within 5.0Å range:
|
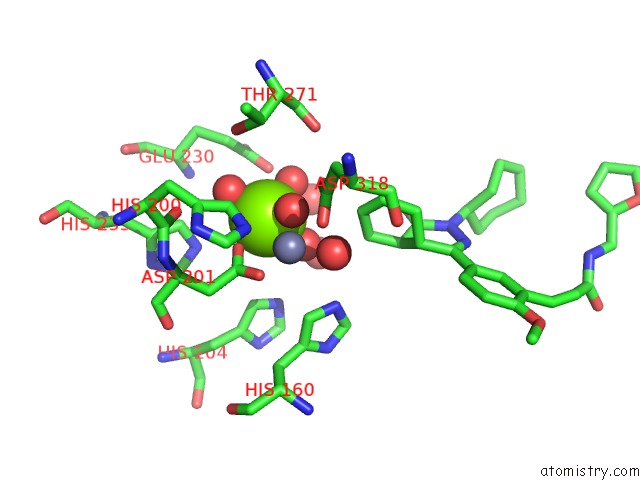
Magnesium binding site 4 out of 4 in 6rcw
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053
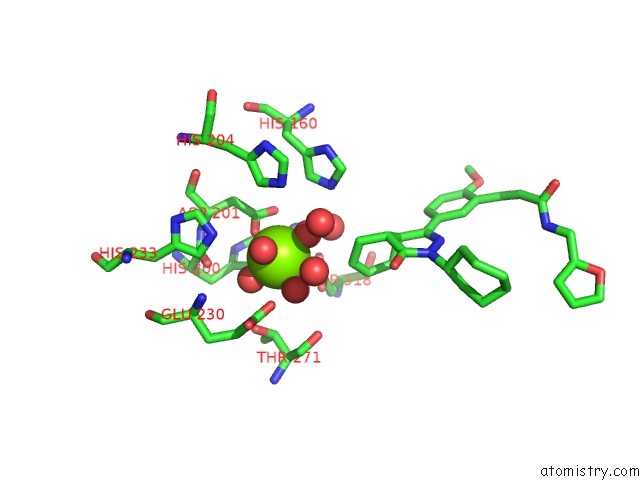
Mono view
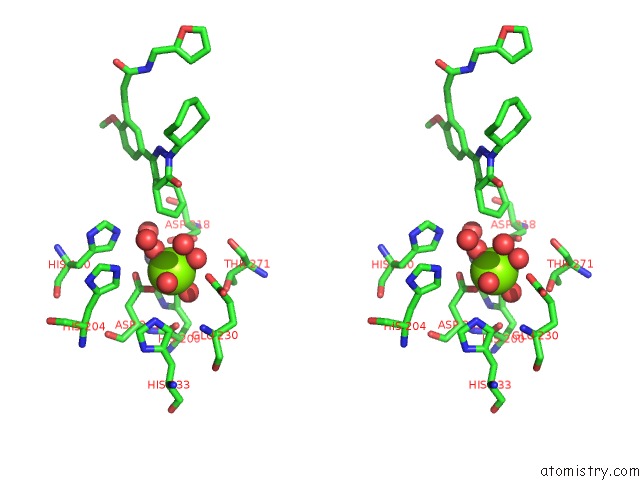
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Human Phosphodiesterase 4D2 Catalytic Domain with Inhibitor Npd-053 within 5.0Å range:
|
Reference:
E.De Heuvel,
A.K.Singh,
P.Boronat,
A.J.Kooistra,
T.Van Der Meer,
P.Sadek,
A.R.Blaazer,
N.C.Shaner,
D.S.Bindels,
G.Caljon,
L.Maes,
G.J.Sterk,
M.Siderius,
M.Oberholzer,
I.J.P.De Esch,
D.G.Brown,
R.Leurs.
Alkynamide Phthalazinones As A New Class of TBRPDEB1 Inhibitors (Part 2). Bioorg.Med.Chem. V. 27 4013 2019.
ISSN: ESSN 1464-3391
PubMed: 31378593
DOI: 10.1016/J.BMC.2019.06.026
Page generated: Tue Oct 1 16:41:48 2024
ISSN: ESSN 1464-3391
PubMed: 31378593
DOI: 10.1016/J.BMC.2019.06.026
Last articles
K in 8DZVK in 8DYD
K in 8E51
K in 8DOD
K in 8DFL
K in 8DHM
K in 8DFB
K in 8DJB
K in 8DF9
K in 8DF7