Magnesium »
PDB 6wwn-6x4b »
6x1q »
Magnesium in PDB 6x1q: 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
Enzymatic activity of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
All present enzymatic activity of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope:
3.2.1.23;
3.2.1.23;
Other elements in 6x1q:
The structure of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope also contains other interesting chemical elements:
| Sodium | (Na) | 8 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
(pdb code 6x1q). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 8 binding sites of Magnesium where determined in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope, PDB code: 6x1q:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Magnesium where determined in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope, PDB code: 6x1q:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Magnesium binding site 1 out of 8 in 6x1q
Go back to
Magnesium binding site 1 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
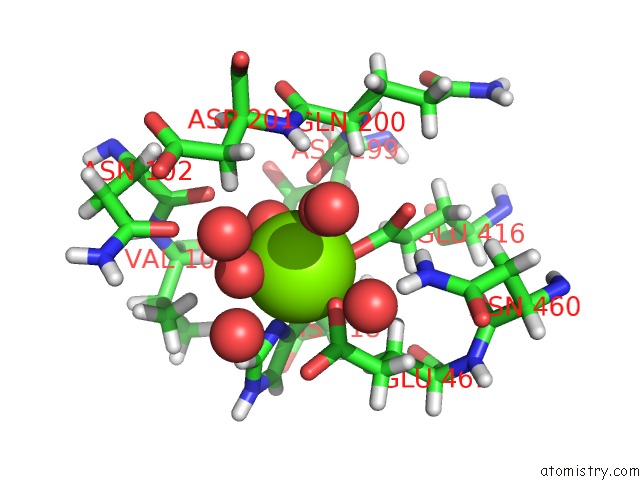
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Magnesium binding site 2 out of 8 in 6x1q
Go back to
Magnesium binding site 2 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Magnesium binding site 3 out of 8 in 6x1q
Go back to
Magnesium binding site 3 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Magnesium binding site 4 out of 8 in 6x1q
Go back to
Magnesium binding site 4 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
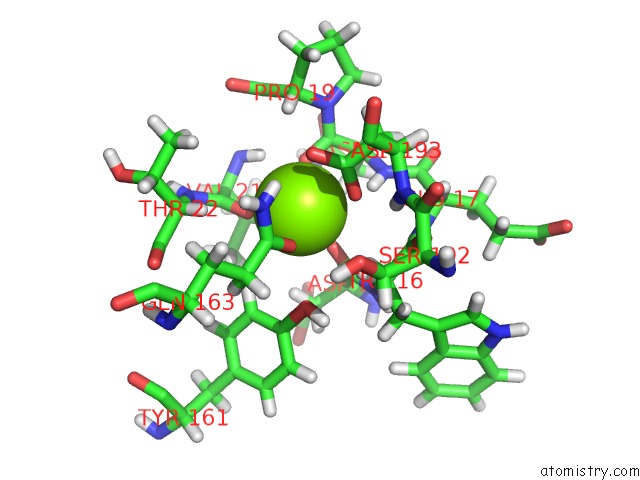
Mono view
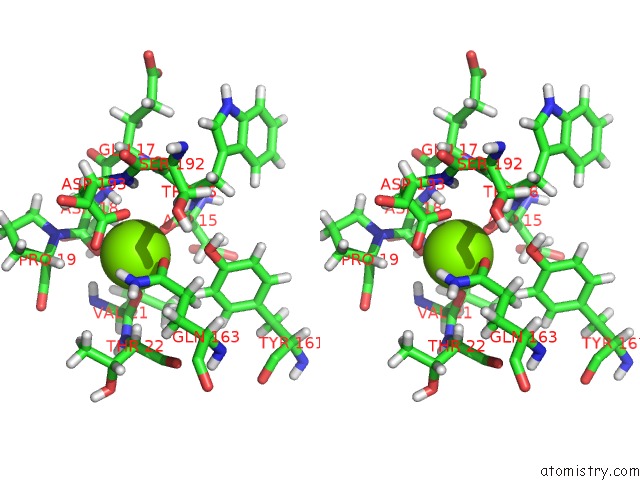
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Magnesium binding site 5 out of 8 in 6x1q
Go back to
Magnesium binding site 5 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
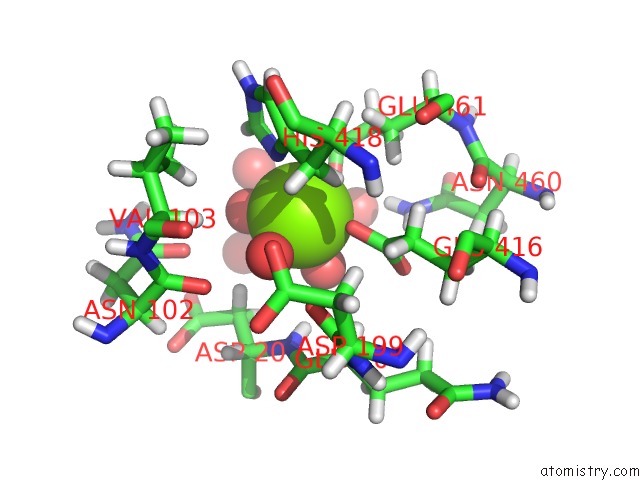
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
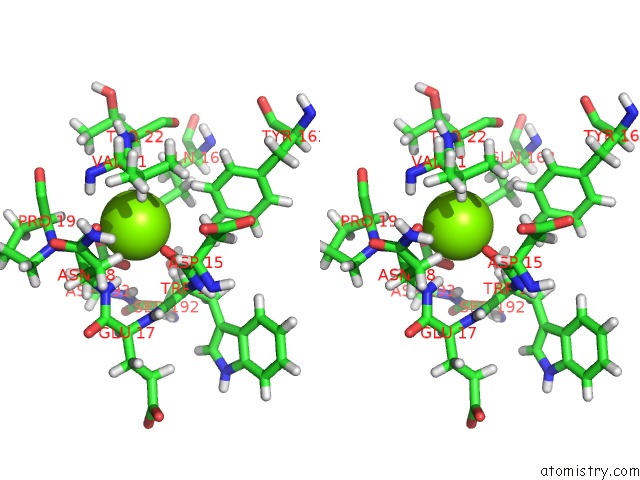
Magnesium binding site 6 out of 8 in 6x1q
Go back to
Magnesium binding site 6 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Magnesium binding site 7 out of 8 in 6x1q
Go back to
Magnesium binding site 7 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
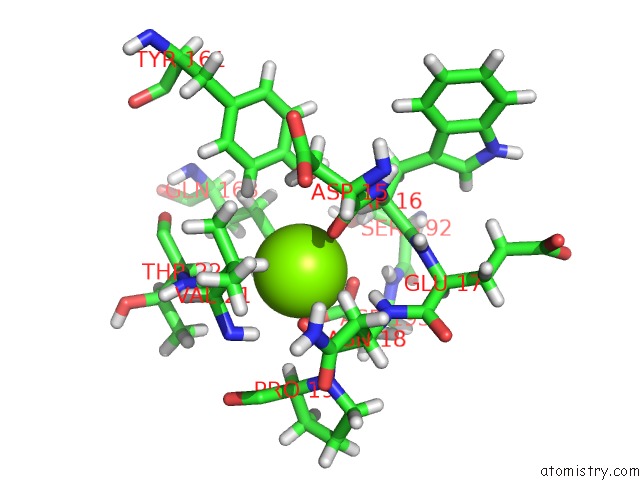
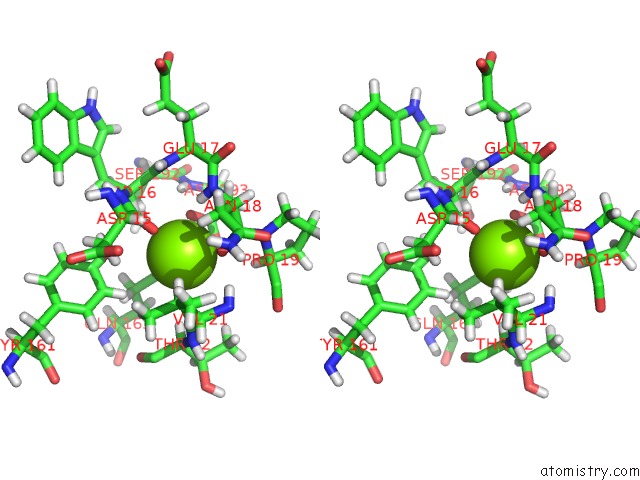
Magnesium binding site 8 out of 8 in 6x1q
Go back to
Magnesium binding site 8 out
of 8 in the 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of 1.8 Angstrom Resolution Structure of B-Galactosidase with A 200 Kv Cryoarm Electron Microscope within 5.0Å range:
|
Reference:
A.Merk,
T.Fukumura,
X.Zhu,
J.E.Darling,
R.Grisshammer,
J.Ognjenovic,
S.Subramaniam.
1.8 Angstrom Resolution Structure of Beta-Galactosidase with A 200 Kv Cryo Arm Electron Microscope. Iucrj V. 7 639 2020.
ISSN: ESSN 2052-2525
PubMed: 32695410
DOI: 10.1107/S2052252520006855
Page generated: Tue Oct 1 23:21:32 2024
ISSN: ESSN 2052-2525
PubMed: 32695410
DOI: 10.1107/S2052252520006855
Last articles
K in 7QR0K in 7QR1
K in 7QQZ
K in 7QQX
K in 7QQW
K in 7QQU
K in 7QQV
K in 7QQT
K in 7QQR
K in 7QQS