Magnesium »
PDB 7ktg-7kyb »
7kwp »
Magnesium in PDB 7kwp: Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
Enzymatic activity of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
All present enzymatic activity of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group:
4.3.3.7;
4.3.3.7;
Protein crystallography data
The structure of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group, PDB code: 7kwp
was solved by
S.Saran,
D.A.R.Sanders,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.84 / 2.26 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 85.4, 231.13, 199.73, 90, 90, 90 |
| R / Rfree (%) | 24.4 / 27.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
(pdb code 7kwp). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group, PDB code: 7kwp:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group, PDB code: 7kwp:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 7kwp
Go back to
Magnesium binding site 1 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
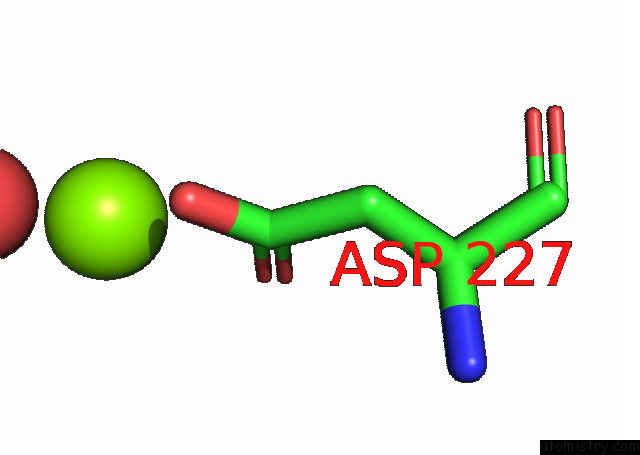
Mono view
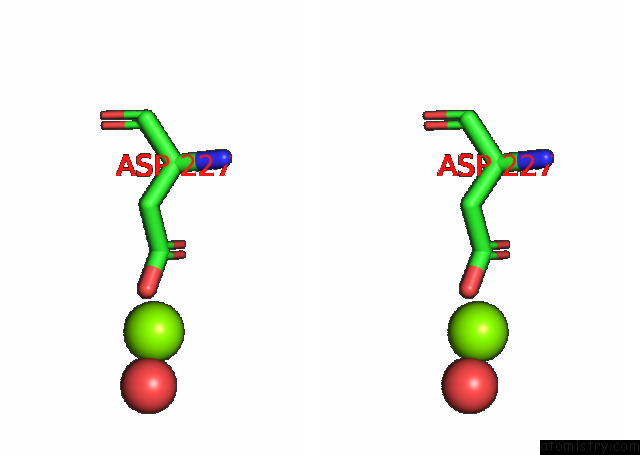
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 7kwp
Go back to
Magnesium binding site 2 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
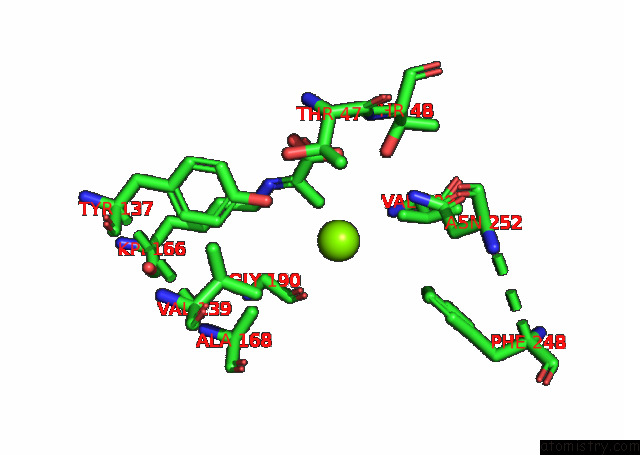
Mono view
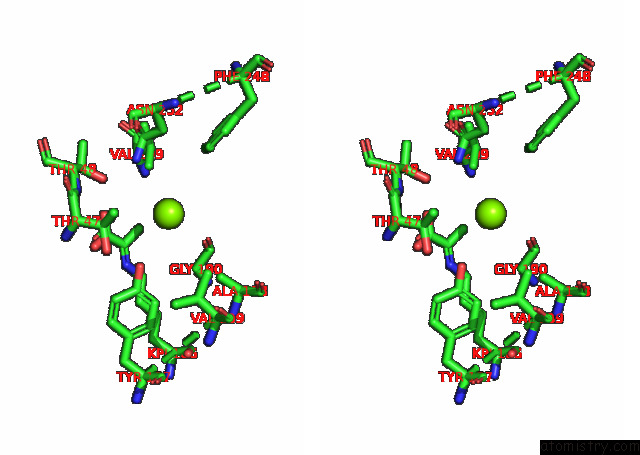
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 7kwp
Go back to
Magnesium binding site 3 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
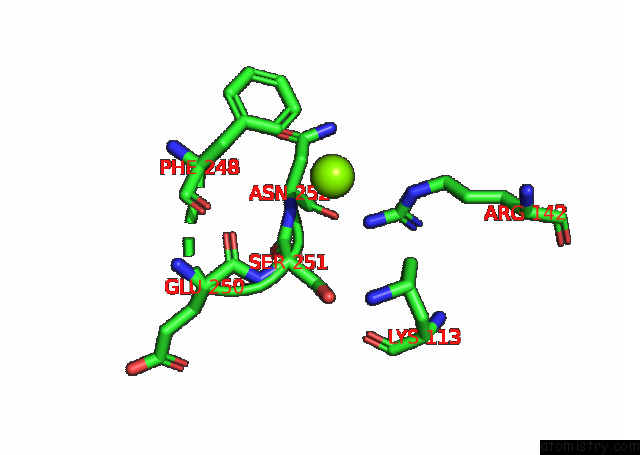
Mono view
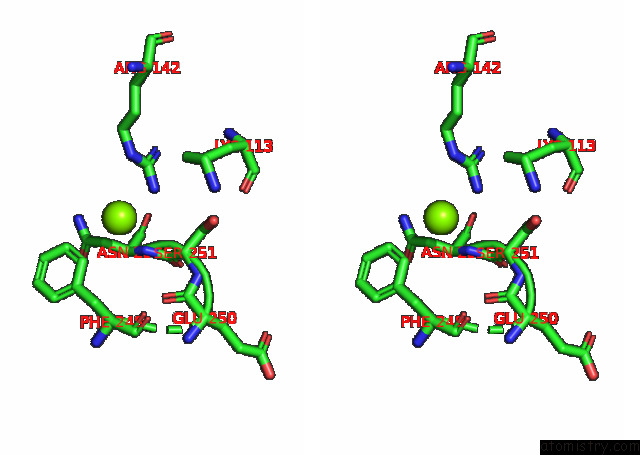
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 7kwp
Go back to
Magnesium binding site 4 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
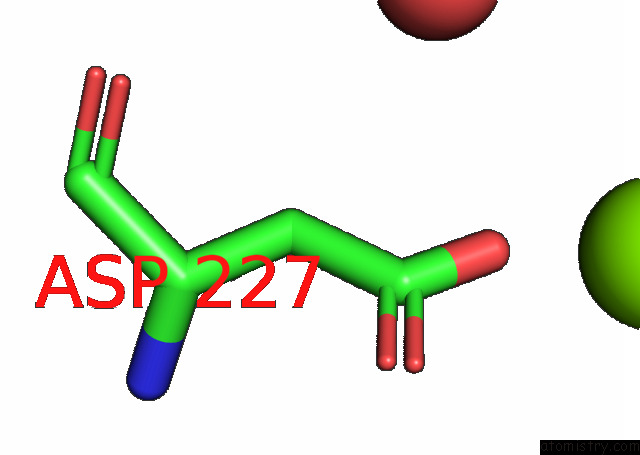
Mono view
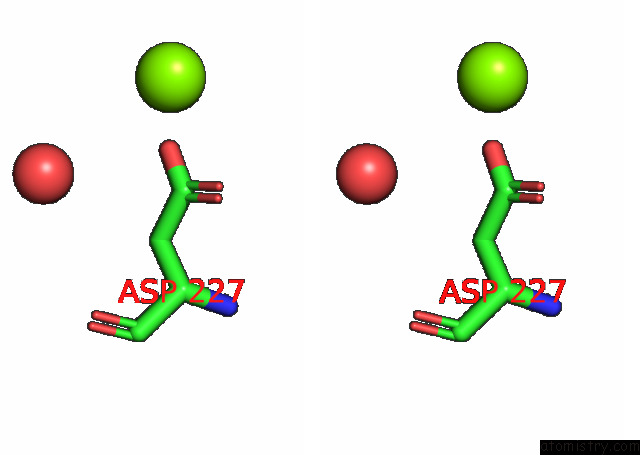
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
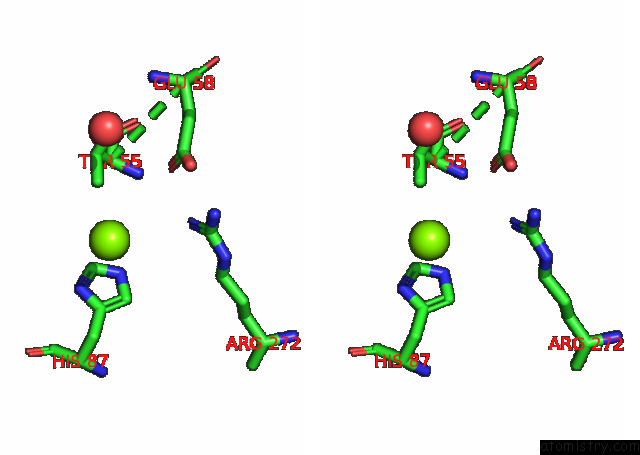
Magnesium binding site 5 out of 6 in 7kwp
Go back to
Magnesium binding site 5 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
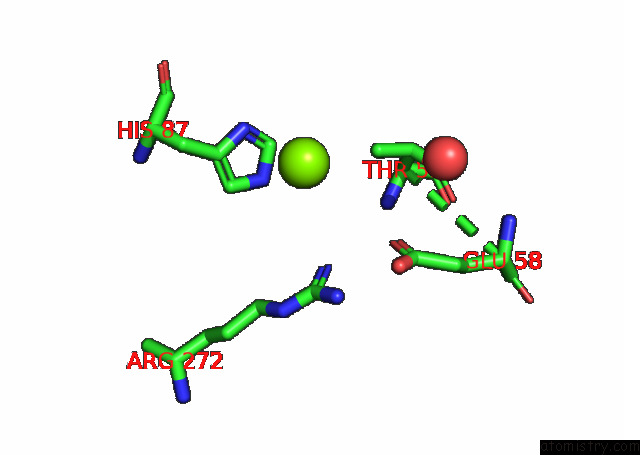
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
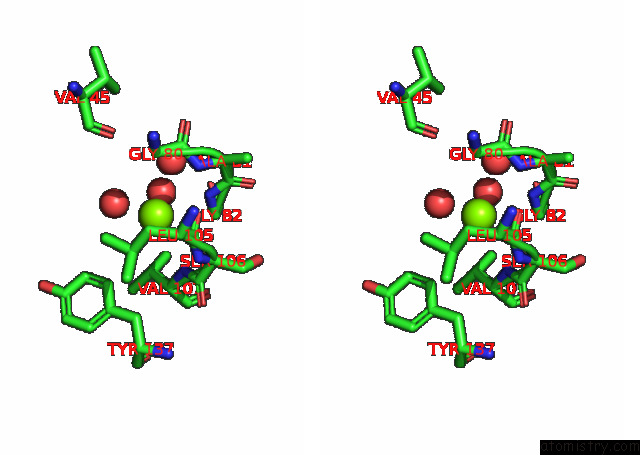
Magnesium binding site 6 out of 6 in 7kwp
Go back to
Magnesium binding site 6 out
of 6 in the Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group
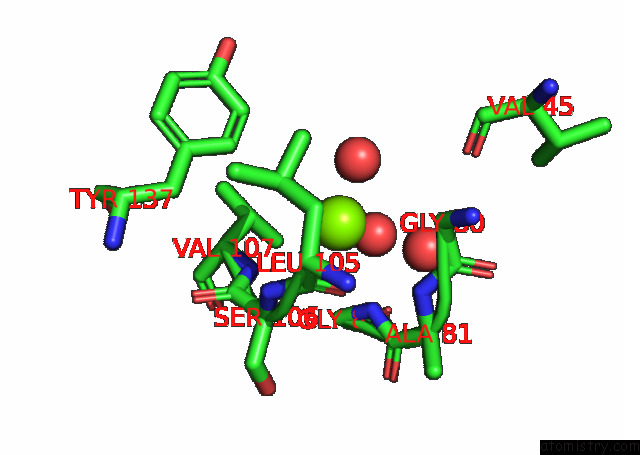
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Dihydrodipicolinate Synthase (Dhdps) From C.Jejuni with Pyruvate Bound in the Active Site and L-Lysine Bound at the Allosteric Site in C2221 Space Group within 5.0Å range:
|
Reference:
S.Saran,
D.A.R.Sanders.
B-Factor Analysis Suggest That L-Lysine and R, R-Bislysine Allosterically Inhibit Cj.Dhdps Enzyme By Decreasing Protein Dynamics. To Be Published.
Page generated: Wed Oct 2 22:57:38 2024
Last articles
K in 6YN2K in 6YLO
K in 6YLN
K in 6Y0T
K in 6YD6
K in 6YD5
K in 6YD1
K in 6Y72
K in 6YAA
K in 6Y3A