Magnesium »
PDB 8u3b-8ucw »
8ucw »
Magnesium in PDB 8ucw: Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase
Enzymatic activity of Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase
All present enzymatic activity of Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase:
2.7.7.7;
2.7.7.7;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase
(pdb code 8ucw). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase, PDB code: 8ucw:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase, PDB code: 8ucw:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8ucw
Go back to
Magnesium binding site 1 out
of 2 in the Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase
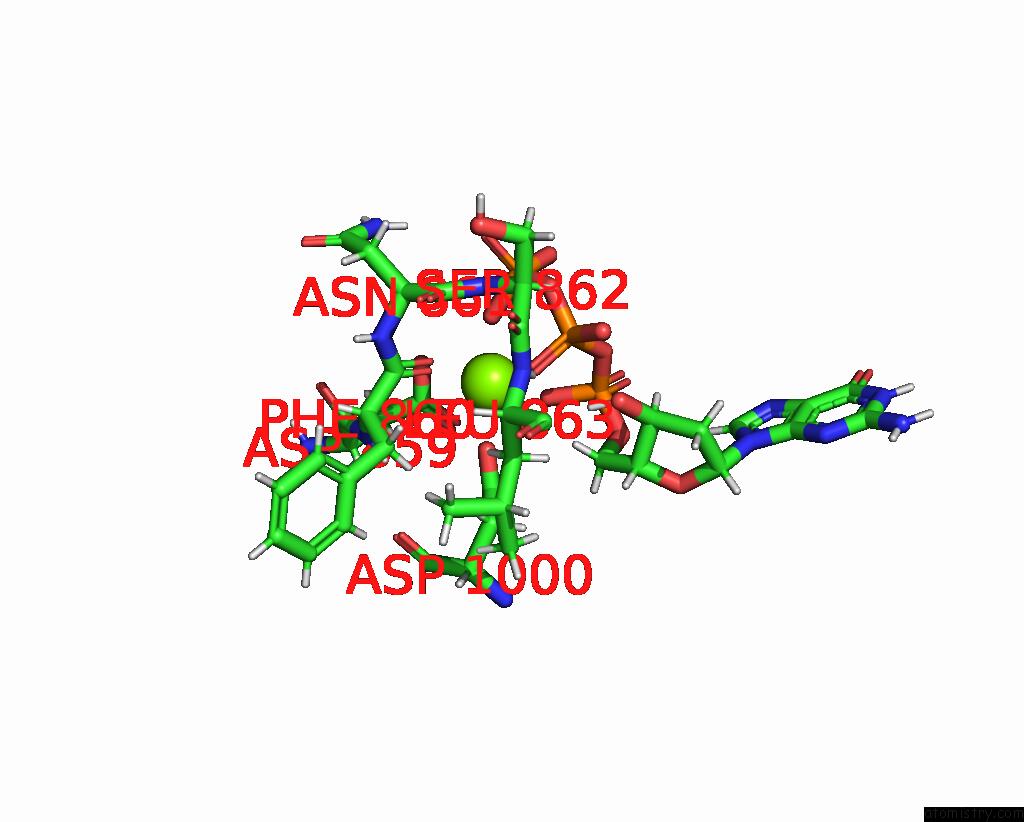
Mono view
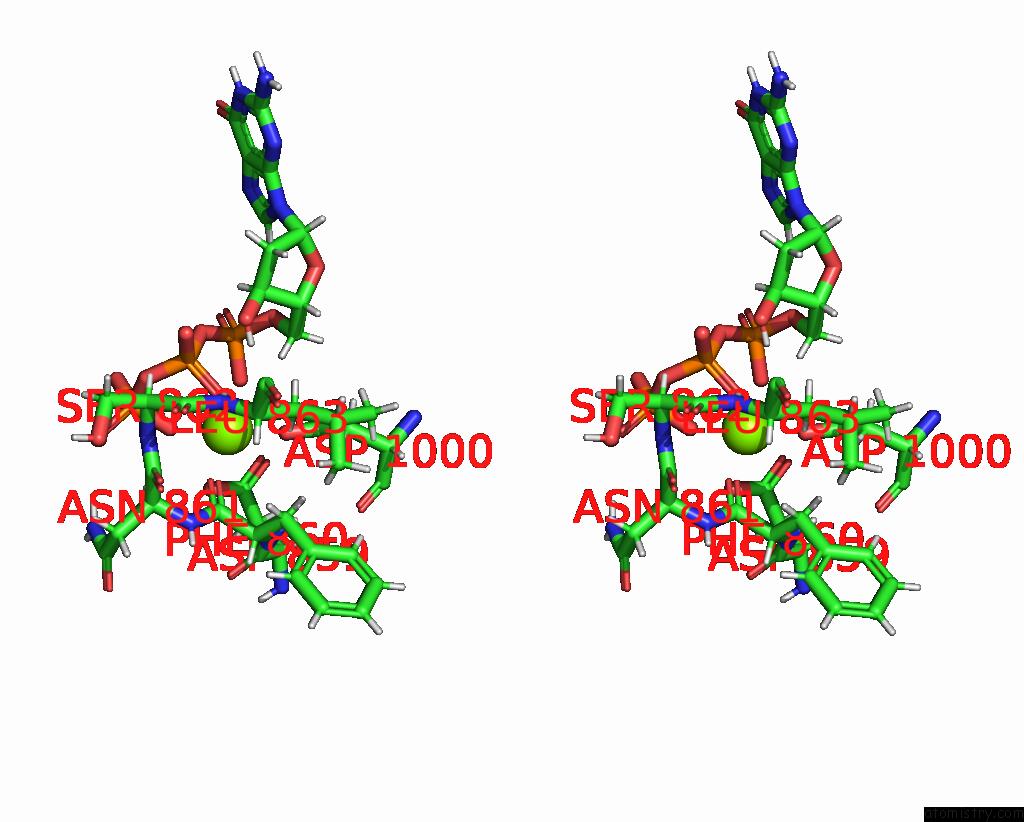
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8ucw
Go back to
Magnesium binding site 2 out
of 2 in the Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase

Mono view
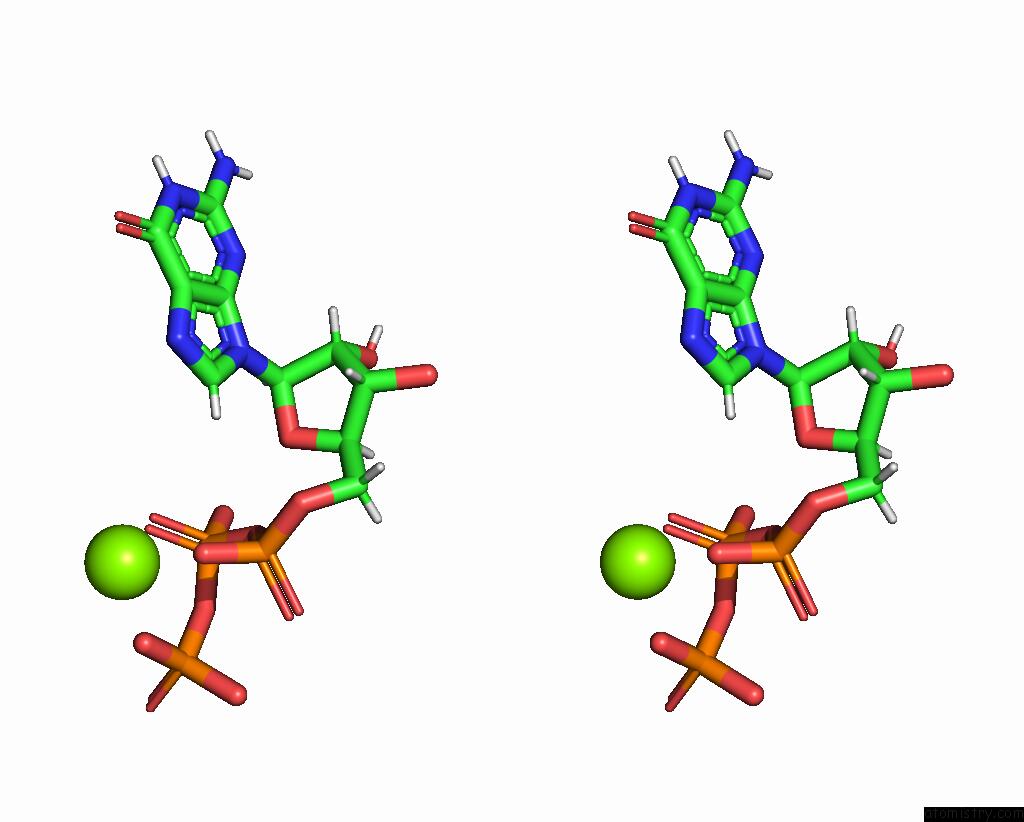
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Complete Dna Termination Subcomplex 2 of Xenopus Laevis Dna Polymerase Alpha-Primase within 5.0Å range:
|
Reference:
E.A.Mullins,
L.E.Salay,
C.L.Durie,
N.P.Bradley,
J.E.Jackman,
M.D.Ohi,
W.J.Chazin,
B.F.Eichman.
A Mechanistic Model of Primer Synthesis From Catalytic Structures of Dna Polymerase Alpha-Primase. Biorxiv 2023.
ISSN: ISSN 2692-8205
PubMed: 36993335
DOI: 10.1101/2023.03.16.533013
Page generated: Fri Aug 15 16:54:37 2025
ISSN: ISSN 2692-8205
PubMed: 36993335
DOI: 10.1101/2023.03.16.533013
Last articles
Mn in 1ZQQMn in 1ZQM
Mn in 1ZQL
Mn in 1ZP9
Mn in 1ZM8
Mn in 1ZOP
Mn in 1ZLZ
Mn in 1ZJP
Mn in 1ZJO
Mn in 1ZDM