Magnesium »
PDB 9b2d-9bm0 »
9bgf »
Magnesium in PDB 9bgf: Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
Enzymatic activity of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
All present enzymatic activity of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac:
2.7.8.17;
2.7.8.17;
Other elements in 9bgf:
The structure of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac also contains other interesting chemical elements:
| Calcium | (Ca) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
(pdb code 9bgf). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac, PDB code: 9bgf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac, PDB code: 9bgf:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 9bgf
Go back to
Magnesium binding site 1 out
of 4 in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
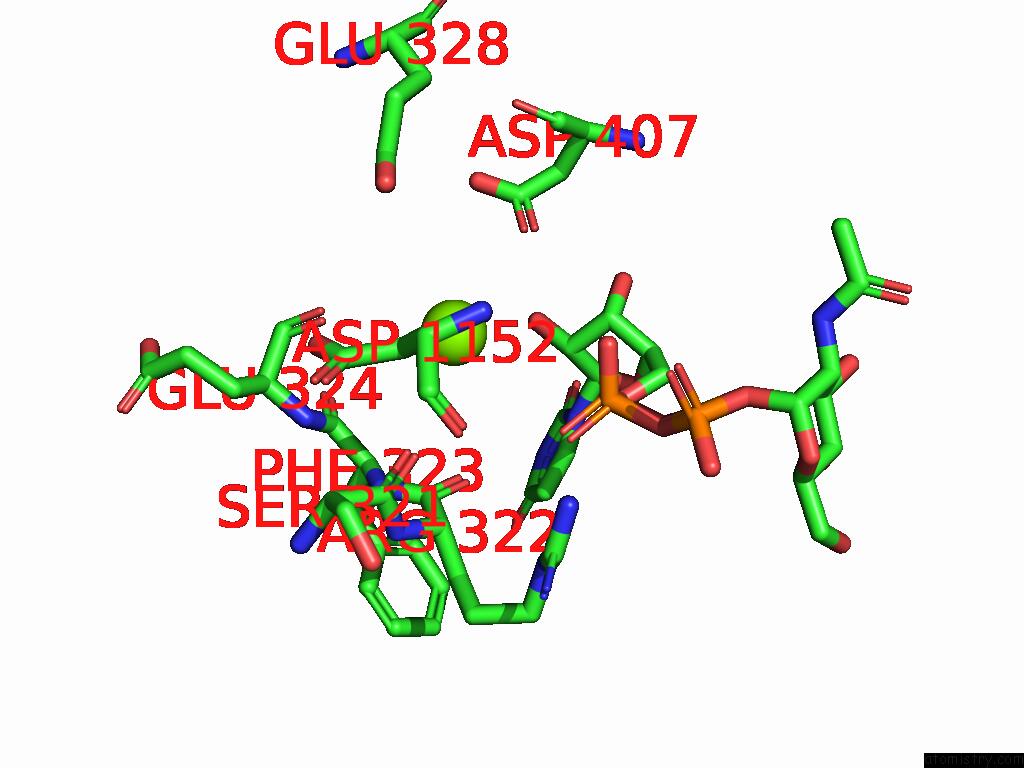
Mono view
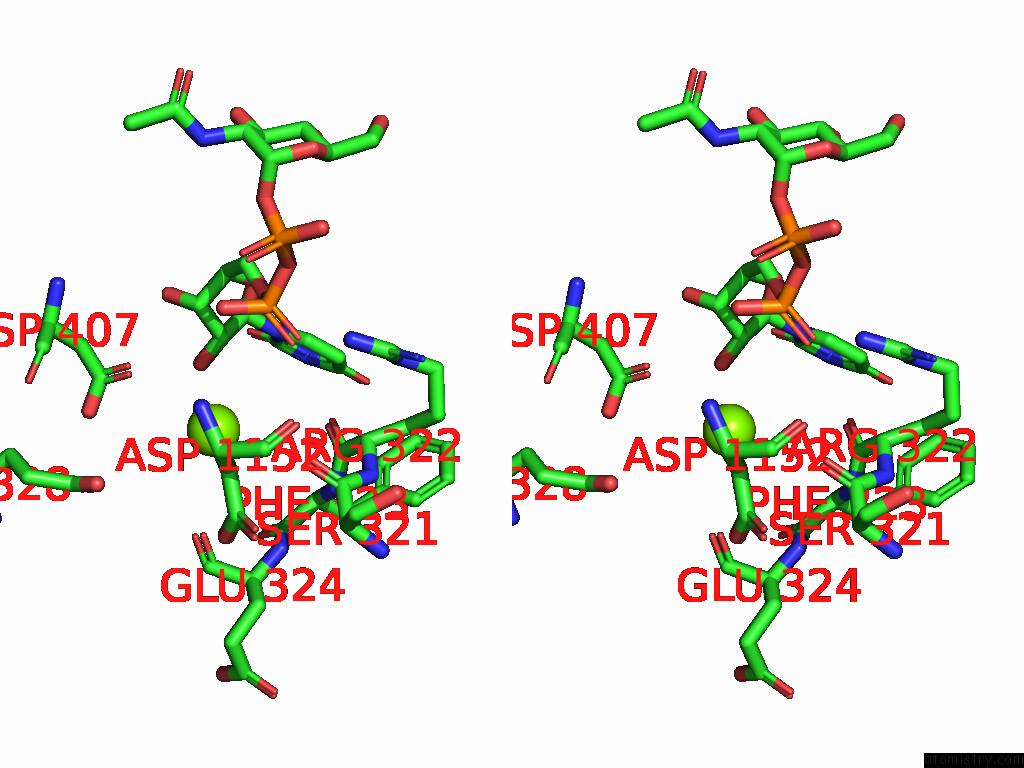
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 9bgf
Go back to
Magnesium binding site 2 out
of 4 in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
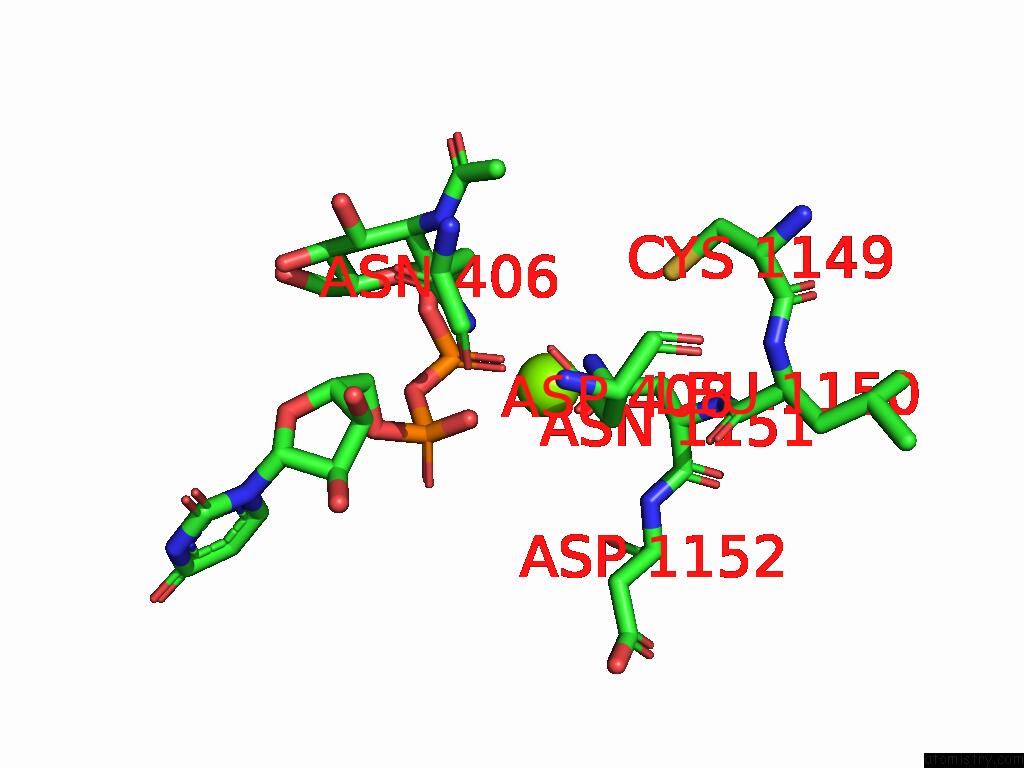
Mono view
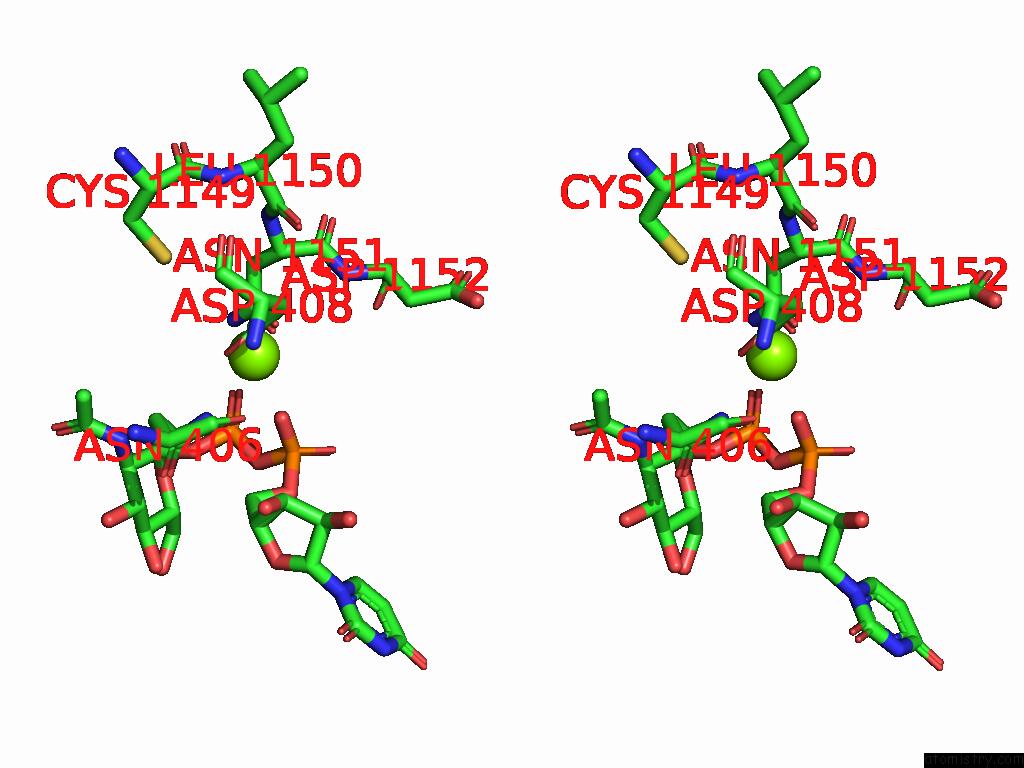
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 9bgf
Go back to
Magnesium binding site 3 out
of 4 in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
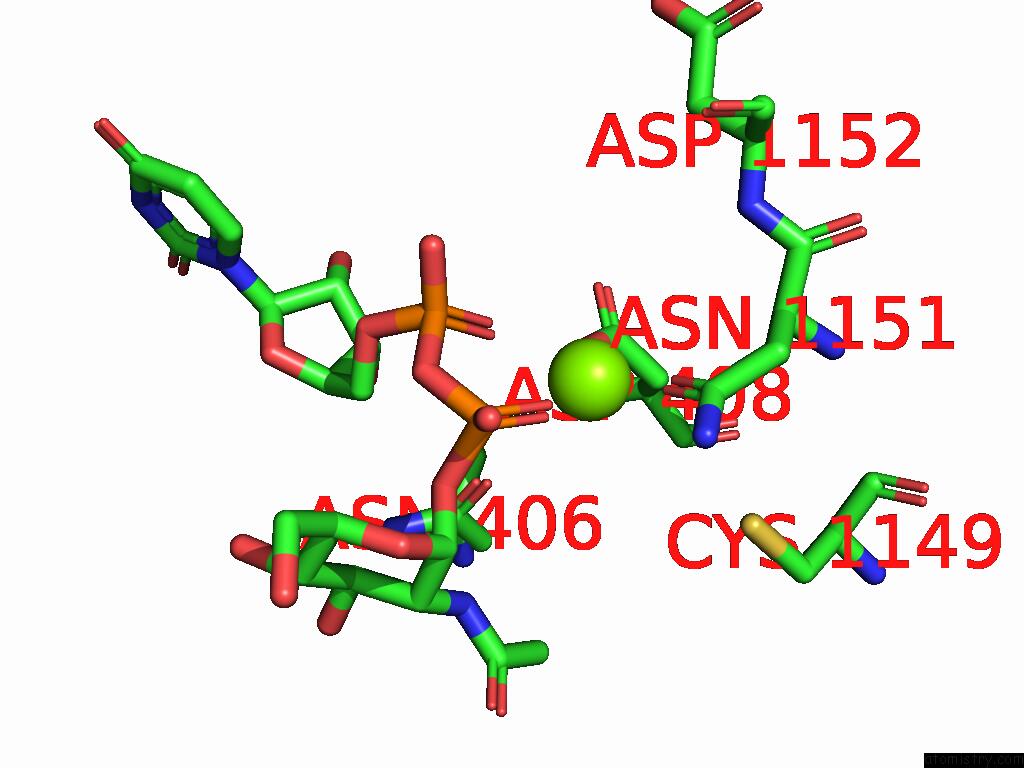
Mono view
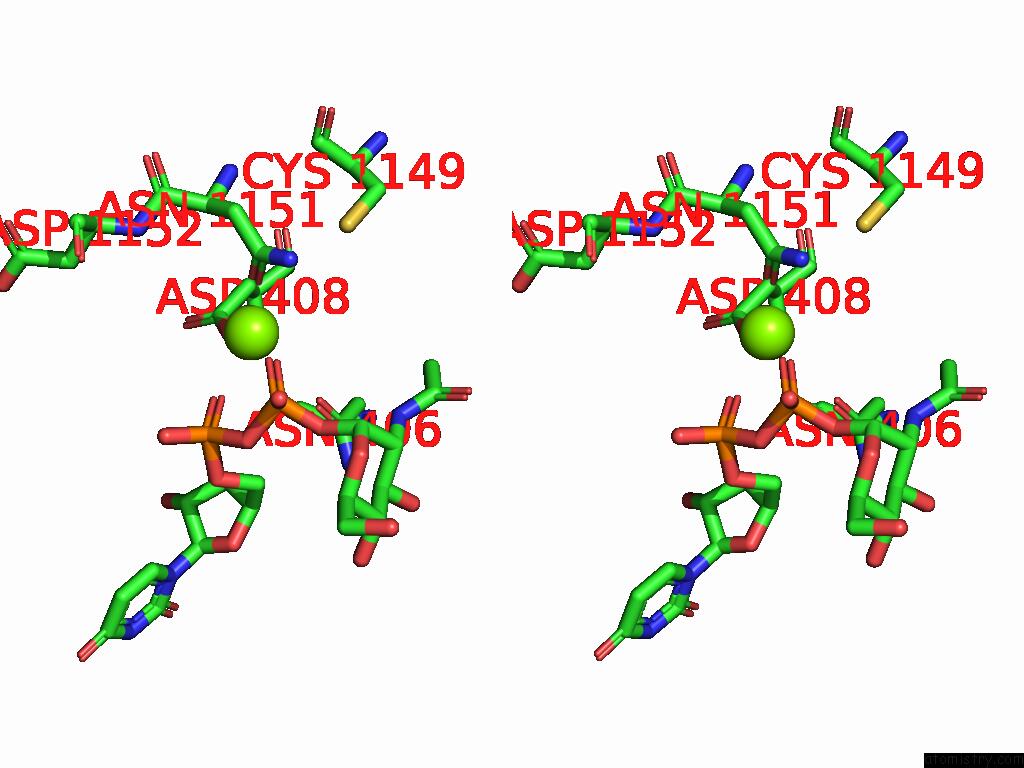
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 9bgf
Go back to
Magnesium binding site 4 out
of 4 in the Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac
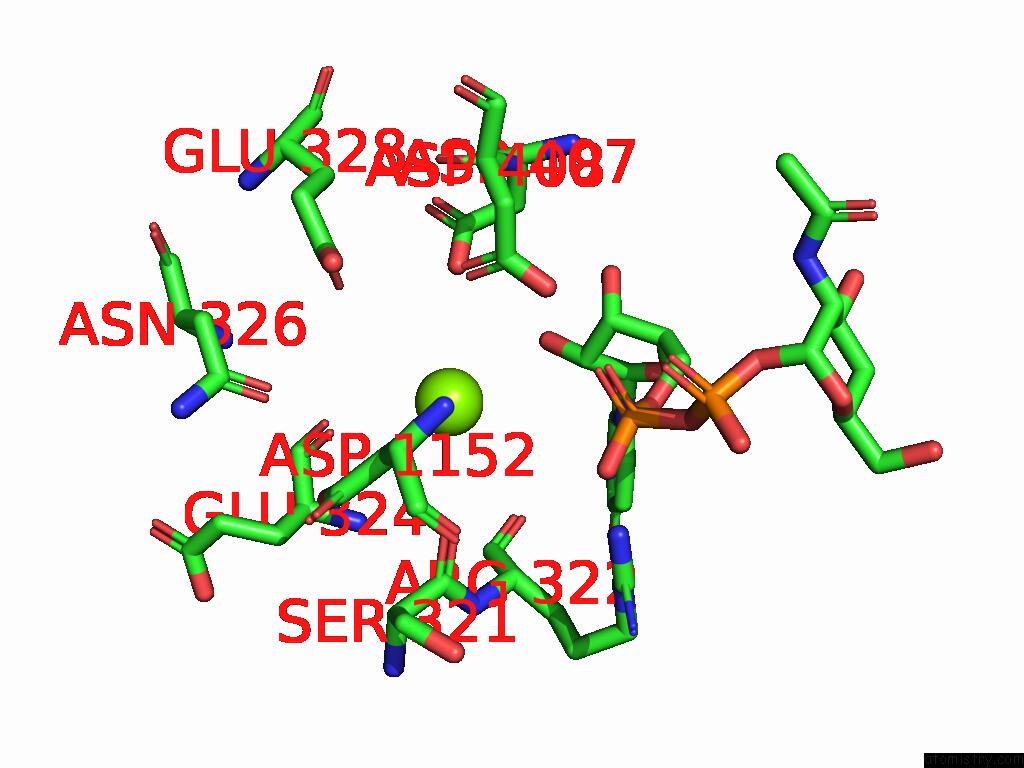
Mono view
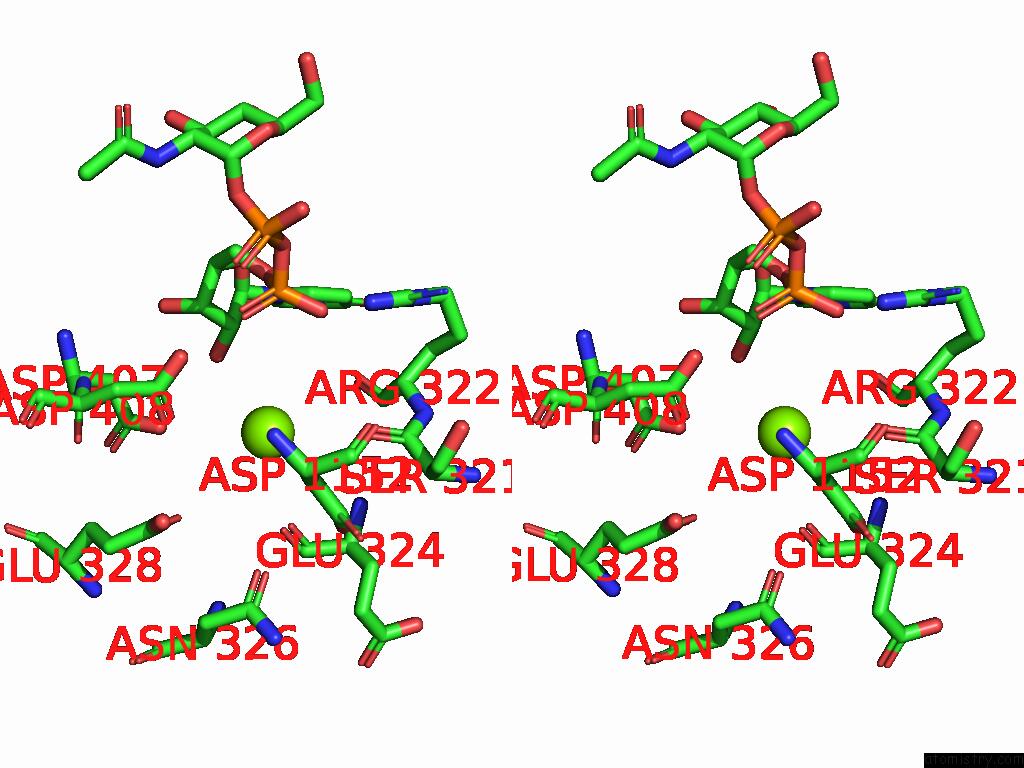
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of Human Glcnac-1-Phosphotransferase Complexed with the Donor Substrate Udp-Glcnac within 5.0Å range:
|
Reference:
H.Li,
B.Doray,
B.C.Jennings,
W.S.Lee,
L.Liu,
S.Kornfeld,
H.Li.
Structure of A Truncated Human Glcnac-1-Phosphotransferase Variant Reveals the Basis For Its Hyperactivity. J.Biol.Chem. V. 300 07706 2024.
ISSN: ESSN 1083-351X
PubMed: 39178950
DOI: 10.1016/J.JBC.2024.107706
Page generated: Fri Aug 15 23:16:31 2025
ISSN: ESSN 1083-351X
PubMed: 39178950
DOI: 10.1016/J.JBC.2024.107706
Last articles
Ni in 4MSANi in 4MS0
Ni in 4MRZ
Ni in 4MRW
Ni in 4MGG
Ni in 4MFG
Ni in 4M76
Ni in 4M6O
Ni in 4M5B
Ni in 4LVN