Magnesium »
PDB 9g23-9gbv »
9g6p »
Magnesium in PDB 9g6p: Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
Enzymatic activity of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
All present enzymatic activity of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds:
5.3.1.5;
5.3.1.5;
Protein crystallography data
The structure of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds, PDB code: 9g6p
was solved by
E.C.Schulz,
A.Prester,
D.V.Stetten,
G.Gore,
C.E.Hatton,
K.Bartels,
J.P.Leimkohl,
H.Schikora,
H.M.Ginn,
F.Tellkamp,
P.Mehrabi,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 71.49 / 1.70 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 94.2, 103.05, 99.25, 90, 90, 90 |
| R / Rfree (%) | 17.4 / 21.5 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
(pdb code 9g6p). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds, PDB code: 9g6p:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds, PDB code: 9g6p:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 9g6p
Go back to
Magnesium binding site 1 out
of 3 in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
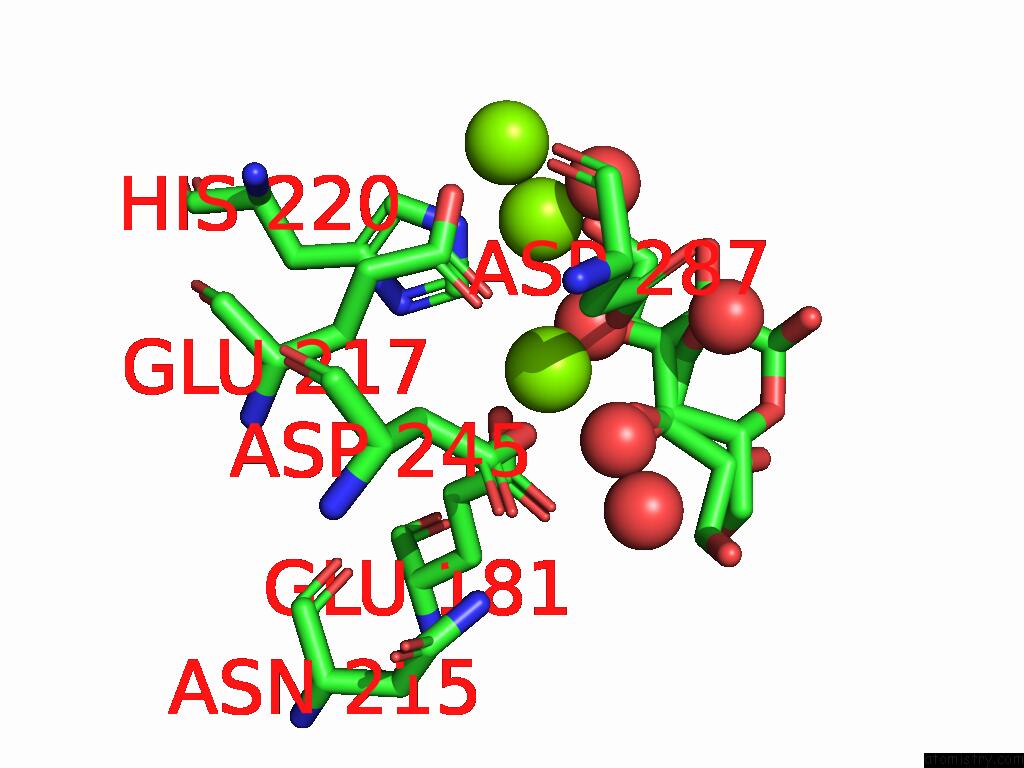
Mono view
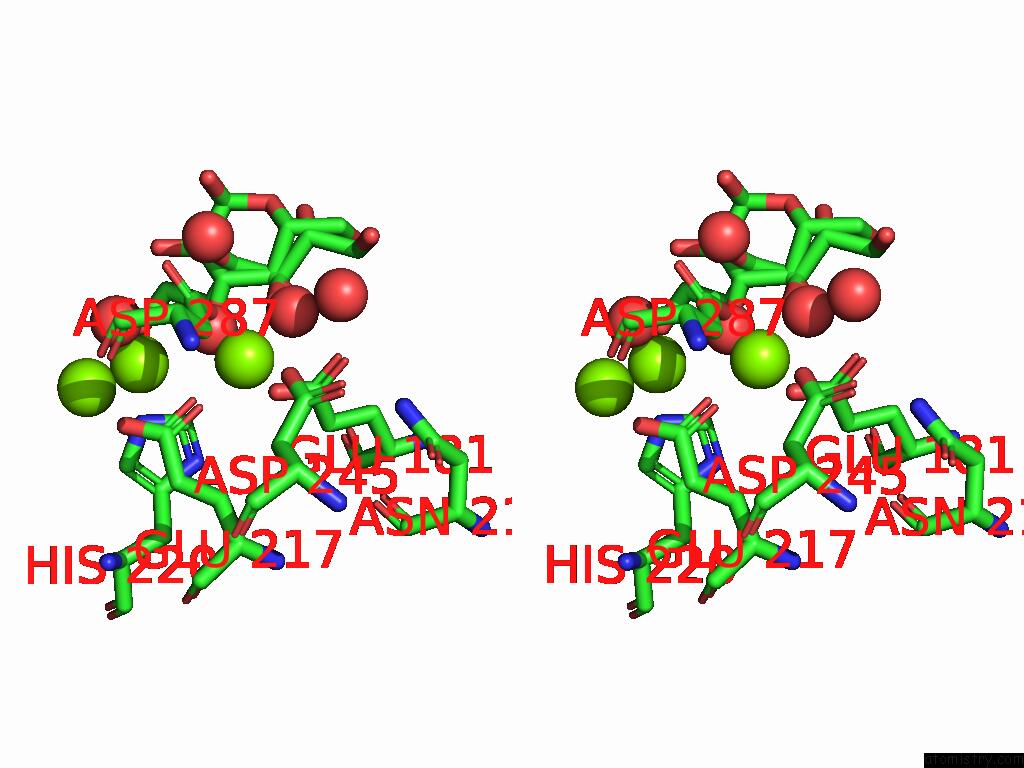
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 9g6p
Go back to
Magnesium binding site 2 out
of 3 in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
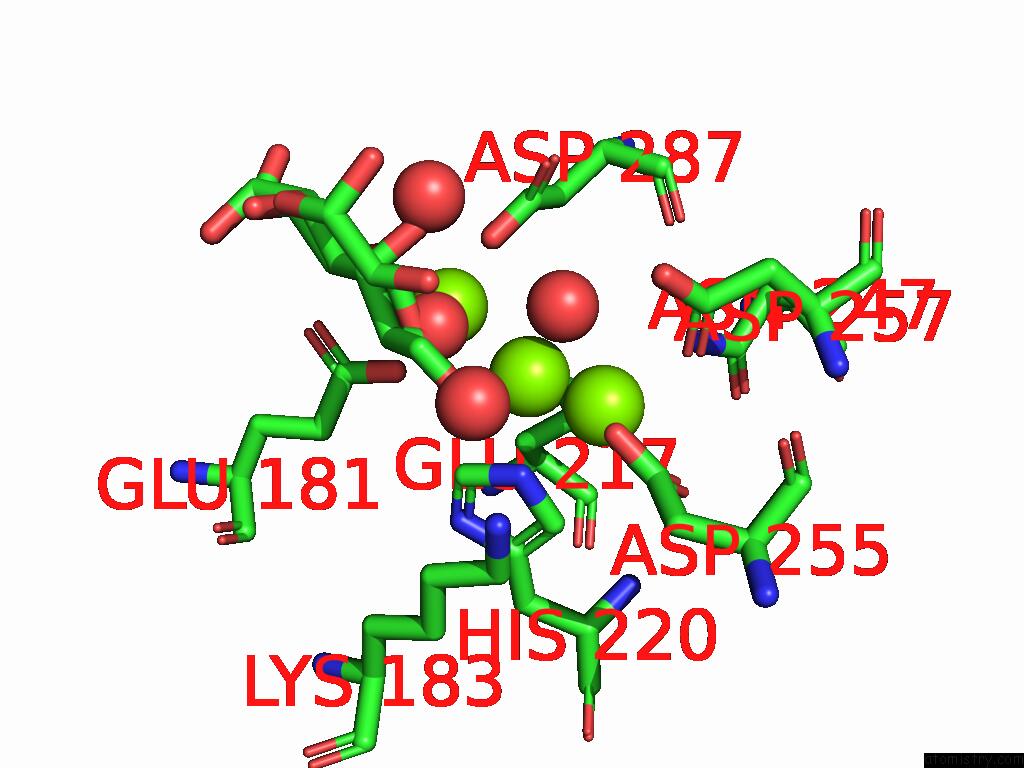
Mono view
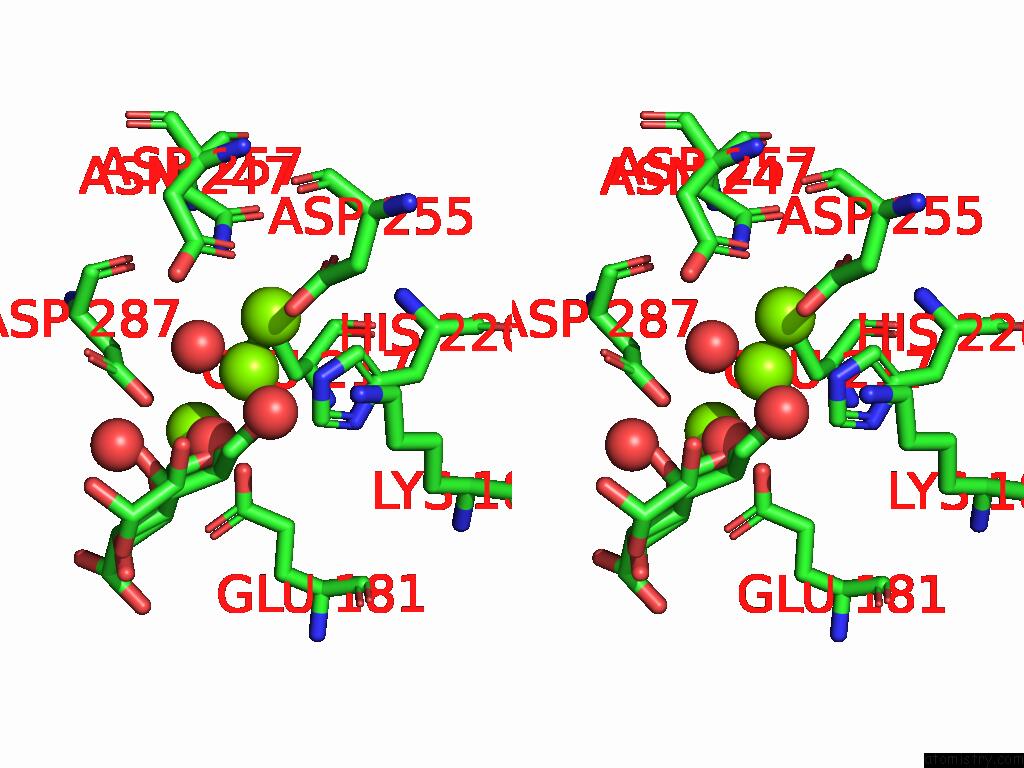
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 9g6p
Go back to
Magnesium binding site 3 out
of 3 in the Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds
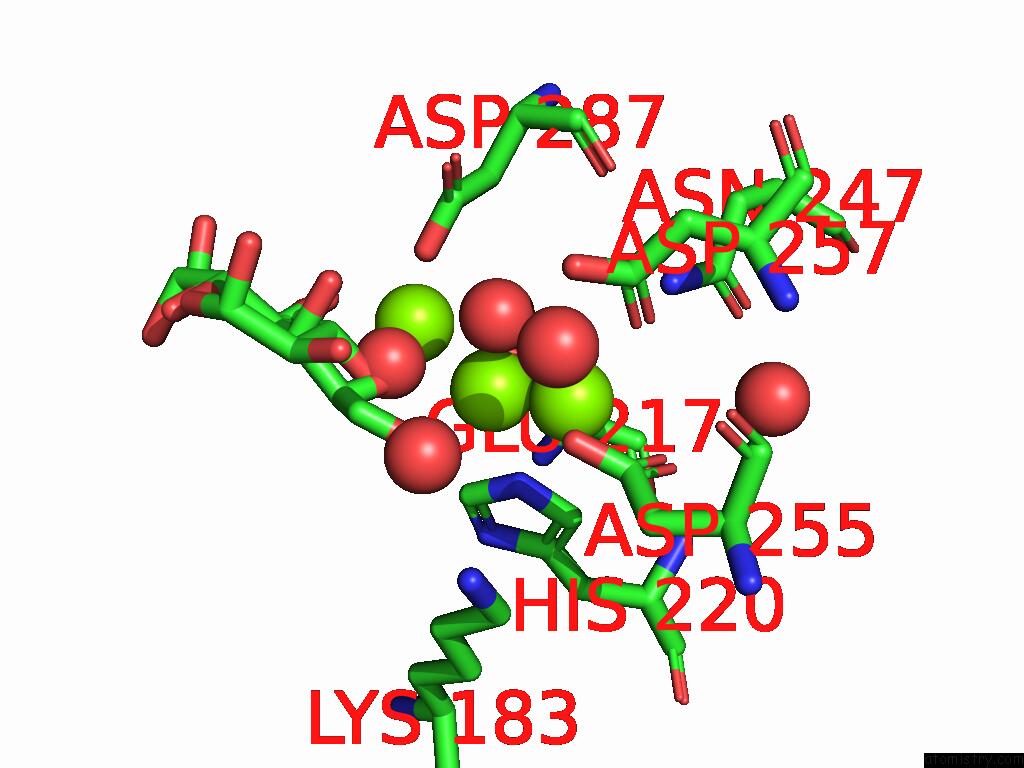
Mono view
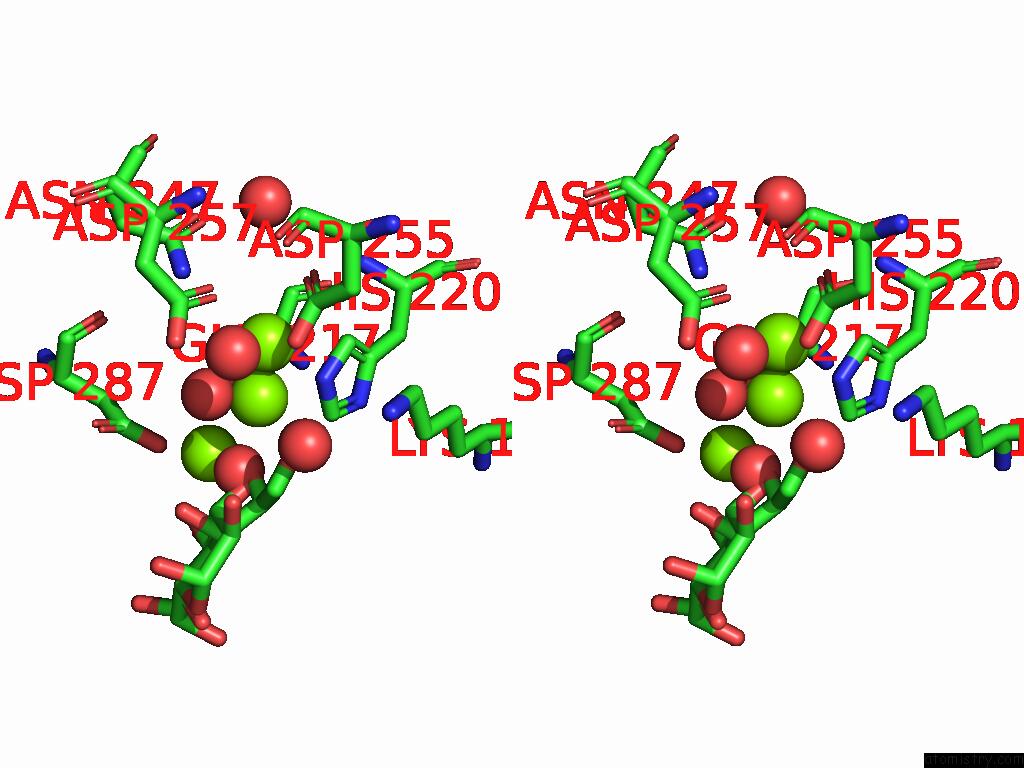
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Xylose Isomerase Collected at 50C Using Time-Resolved Serial Synchrotron Crystallography with Glucose at 60 Seconds within 5.0Å range:
|
Reference:
E.C.Schulz,
A.Prester,
D.Von Stetten,
G.Gore,
C.E.Hatton,
K.Bartels,
J.P.Leimkohl,
H.Schikora,
H.M.Ginn,
F.Tellkamp,
P.Mehrabi.
Probing the Modulation of Enzyme Kinetics By Multi-Temperature, Time-Resolved Serial Crystallography. Nat Commun V. 16 6553 2025.
ISSN: ESSN 2041-1723
PubMed: 40670369
DOI: 10.1038/S41467-025-61631-2
Page generated: Sat Aug 16 02:55:36 2025
ISSN: ESSN 2041-1723
PubMed: 40670369
DOI: 10.1038/S41467-025-61631-2
Last articles
Mn in 4KRVMn in 4KLT
Mn in 4KP7
Mn in 4KMF
Mn in 4KLU
Mn in 4KIR
Mn in 4KLS
Mn in 4KLH
Mn in 4KLQ
Mn in 4KIL