Magnesium »
PDB 9hdr-9ij1 »
9ibe »
Magnesium in PDB 9ibe: D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
Protein crystallography data
The structure of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride, PDB code: 9ibe
was solved by
P.J.Baker,
J.R.Barrett,
A.A.A.B.Dakhil,
J.Domenech,
C.Bisson,
N.Pramanpol,
J.Ferrer,
D.W.Rice,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 52.28 / 1.26 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 66.633, 75.34, 78.234, 108.84, 108.08, 95.53 |
| R / Rfree (%) | 15.2 / 18.8 |
Other elements in 9ibe:
The structure of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride also contains other interesting chemical elements:
| Potassium | (K) | 24 atoms |
| Chlorine | (Cl) | 5 atoms |
Magnesium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 12;Binding sites:
The binding sites of Magnesium atom in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride (pdb code 9ibe). This binding sites where shown within 5.0 Angstroms radius around Magnesium atom.In total 12 binding sites of Magnesium where determined in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride, PDB code: 9ibe:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
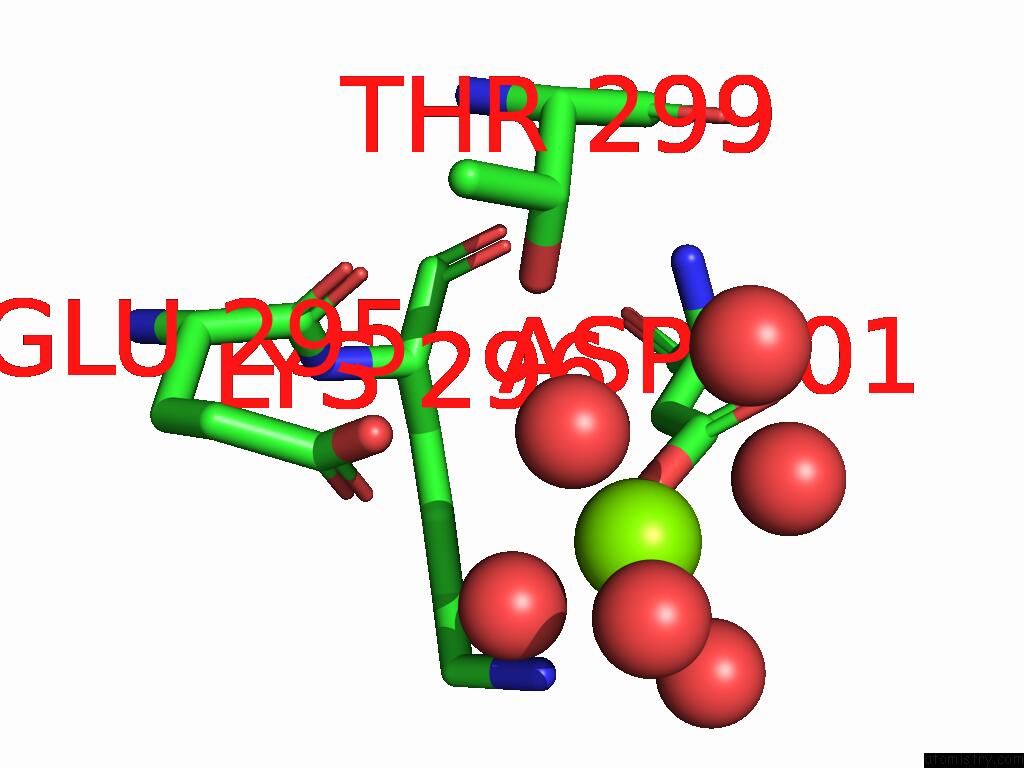
Magnesium binding site 1 out of 12 in 9ibe
Go back to
Magnesium binding site 1 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
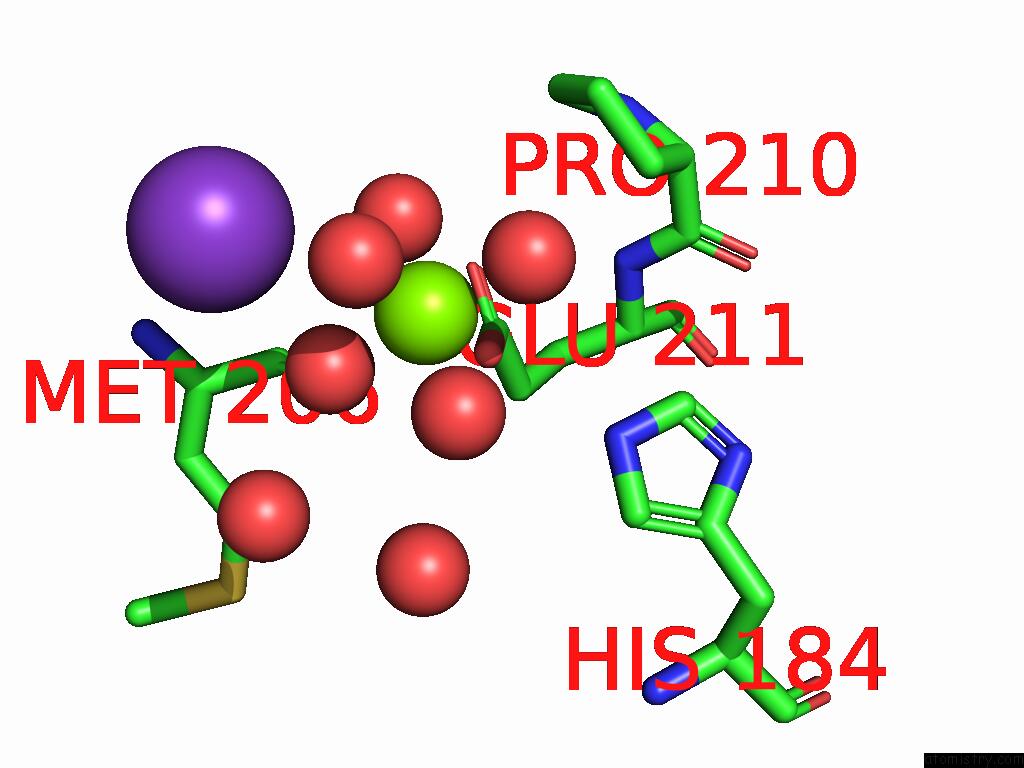
Mono view
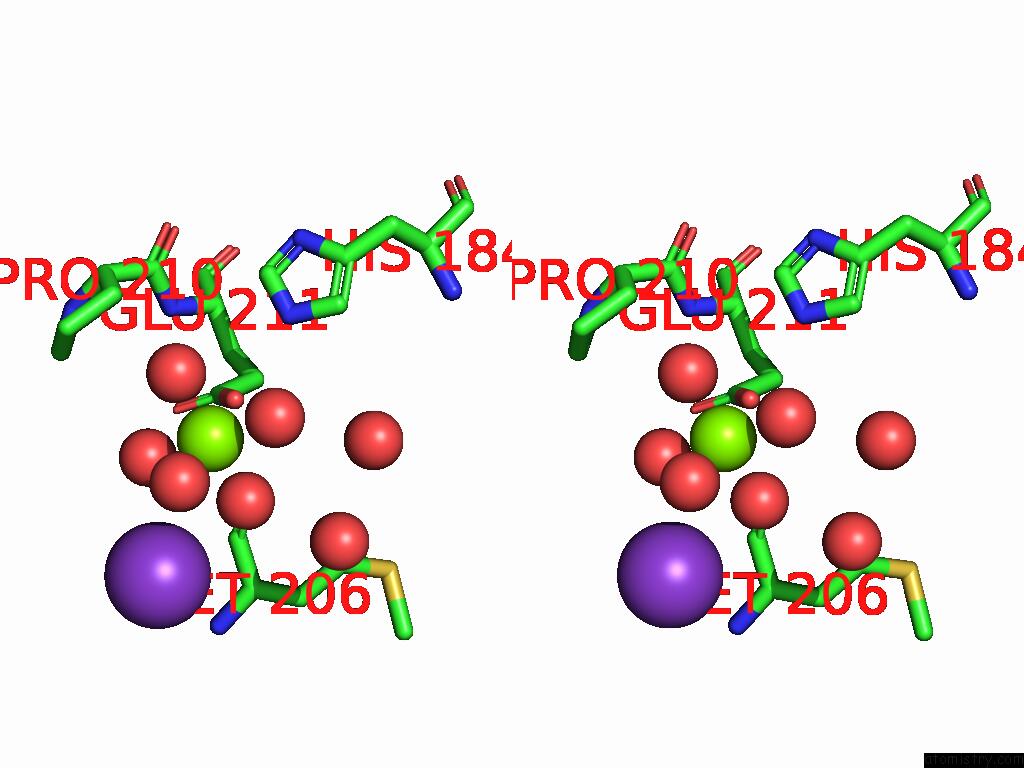
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
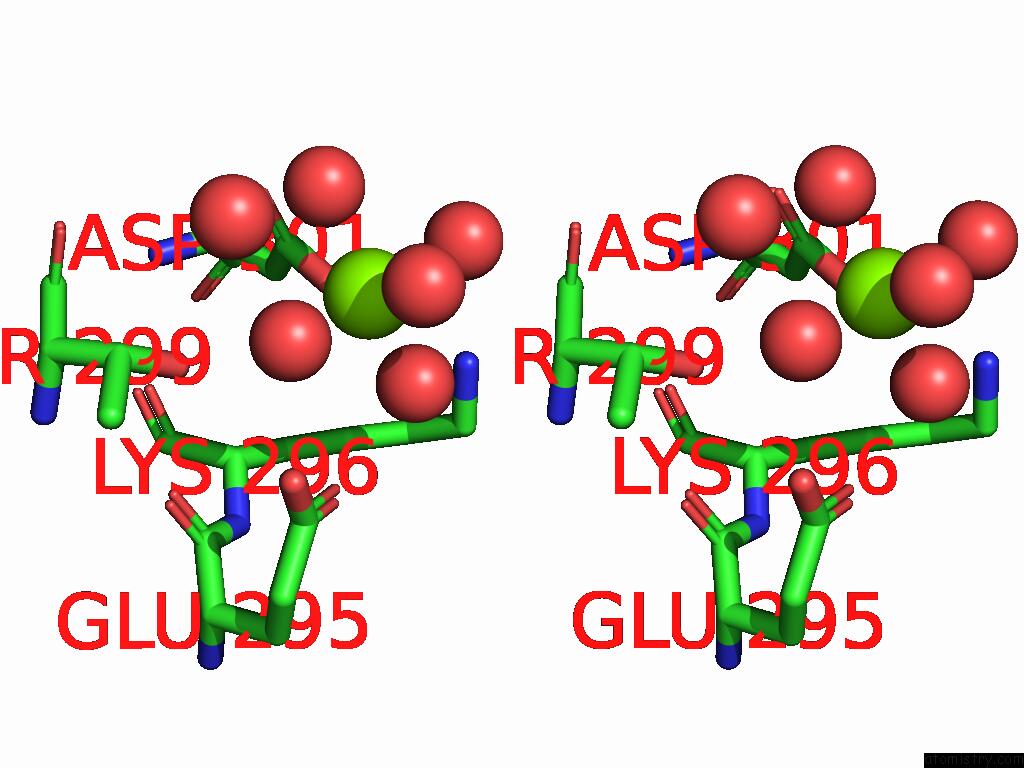
Magnesium binding site 2 out of 12 in 9ibe
Go back to
Magnesium binding site 2 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
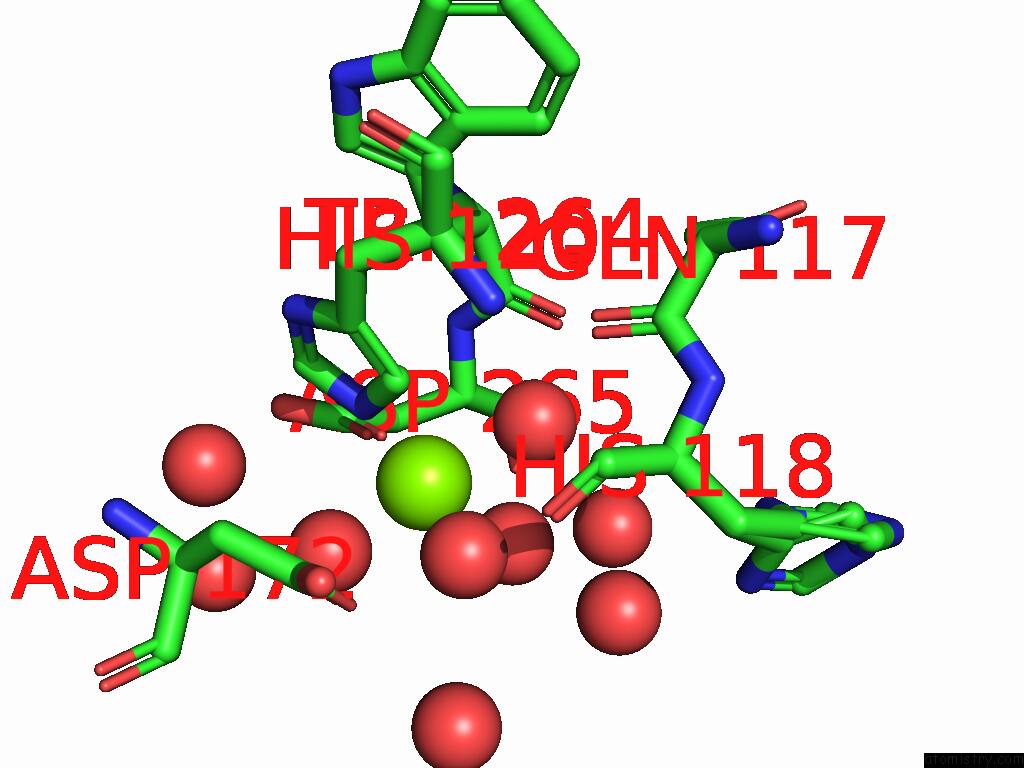
Mono view
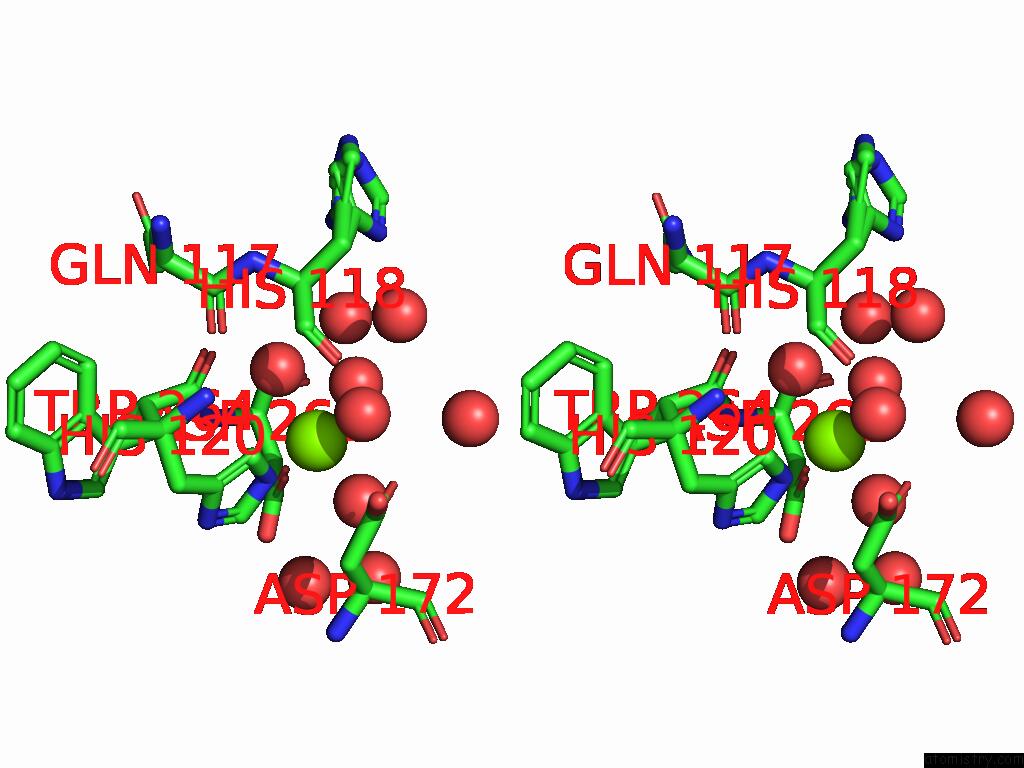
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
Magnesium binding site 3 out of 12 in 9ibe
Go back to
Magnesium binding site 3 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
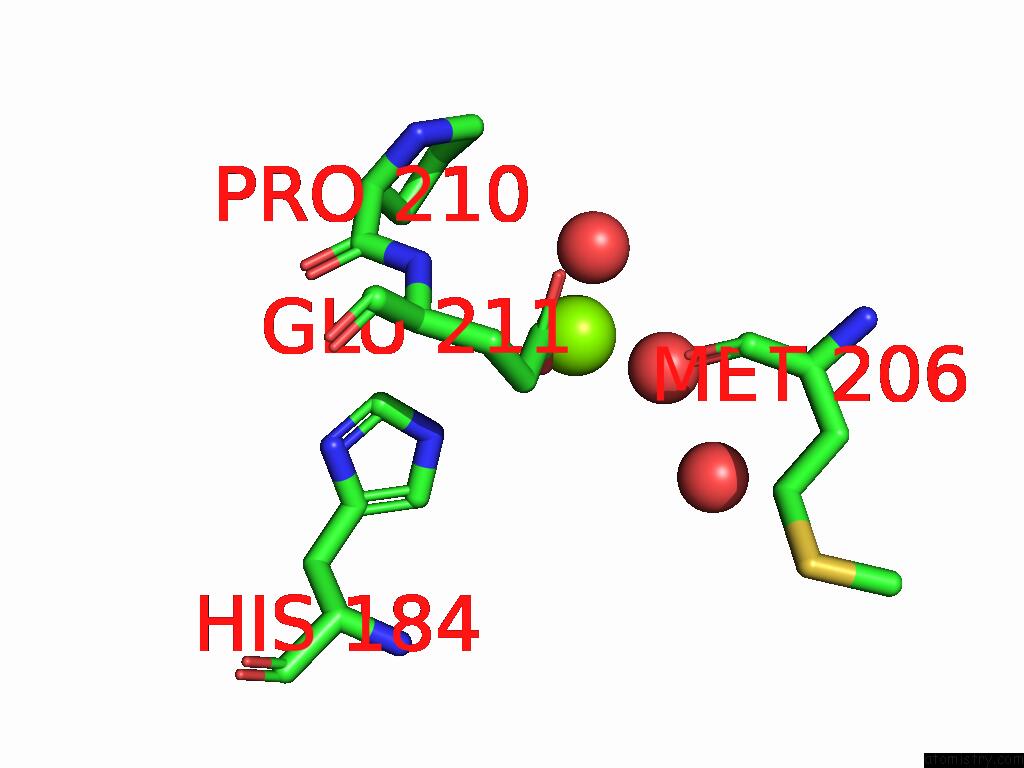
Mono view
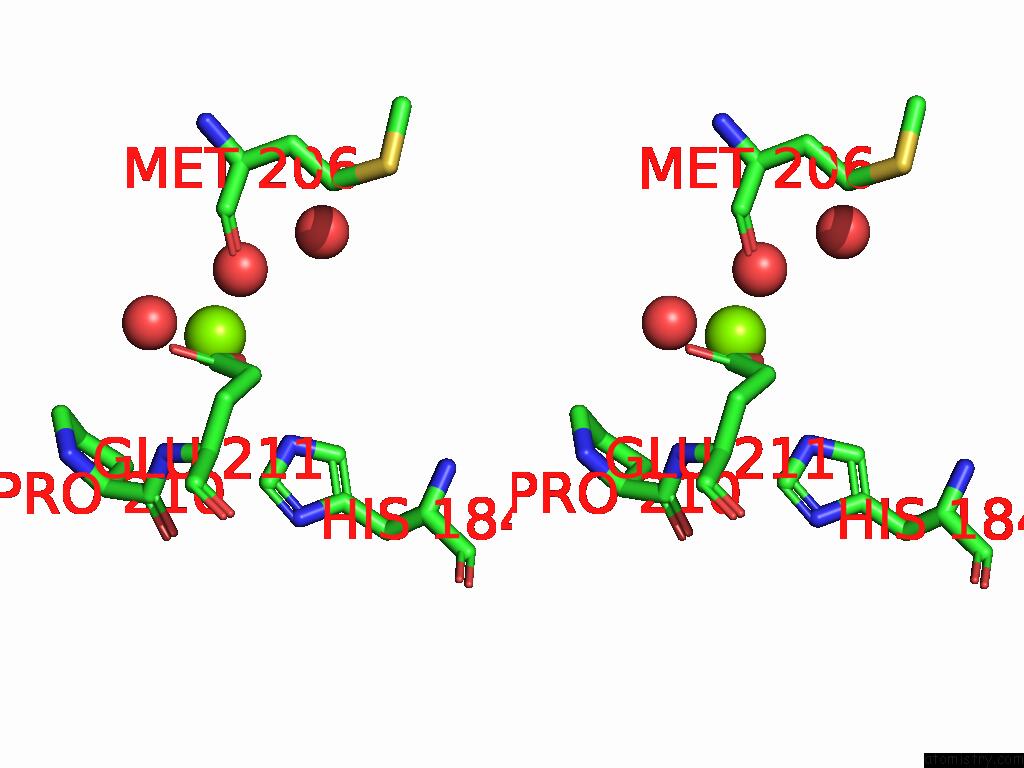
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
Magnesium binding site 4 out of 12 in 9ibe
Go back to
Magnesium binding site 4 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
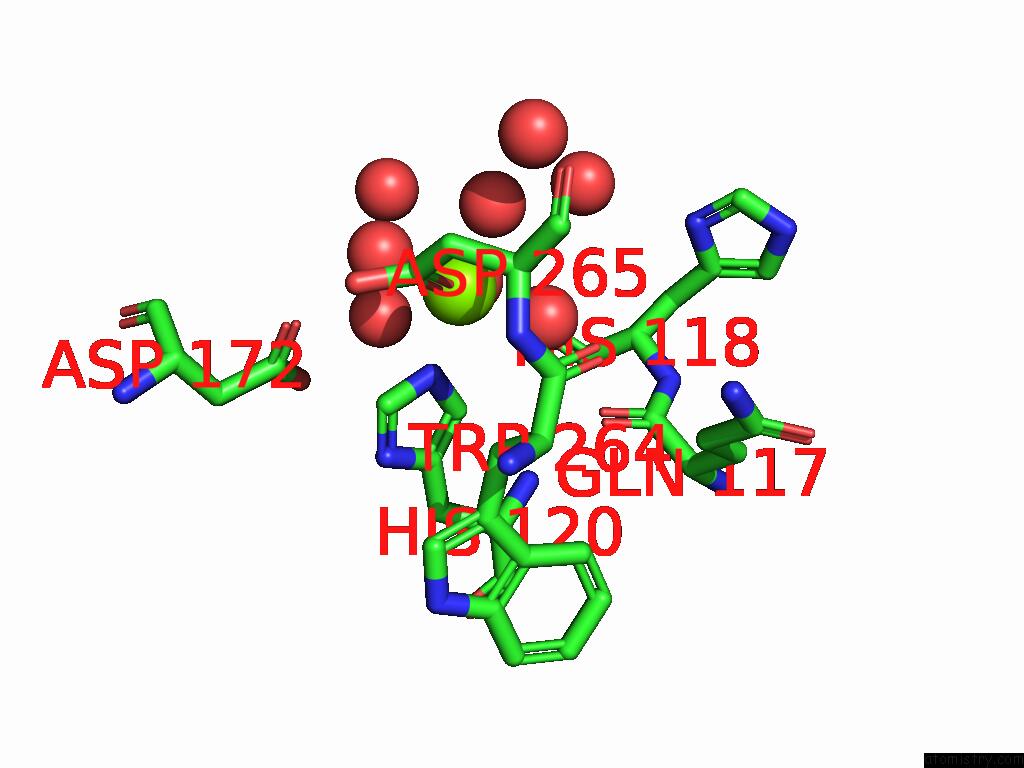
Mono view
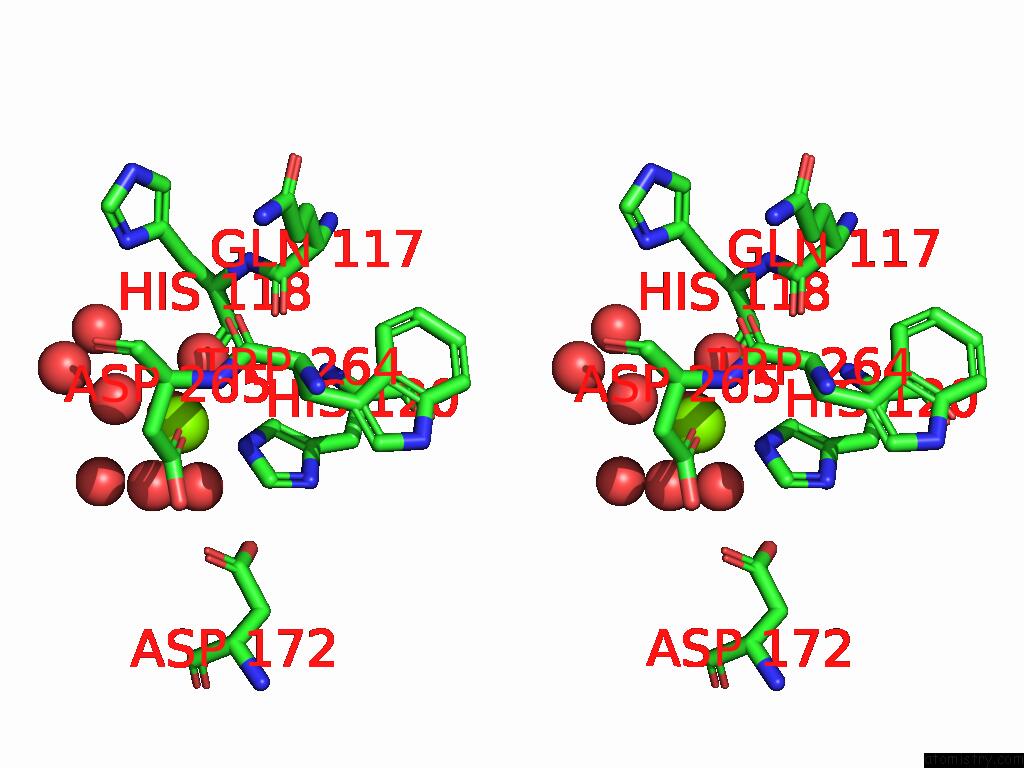
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
Magnesium binding site 5 out of 12 in 9ibe
Go back to
Magnesium binding site 5 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
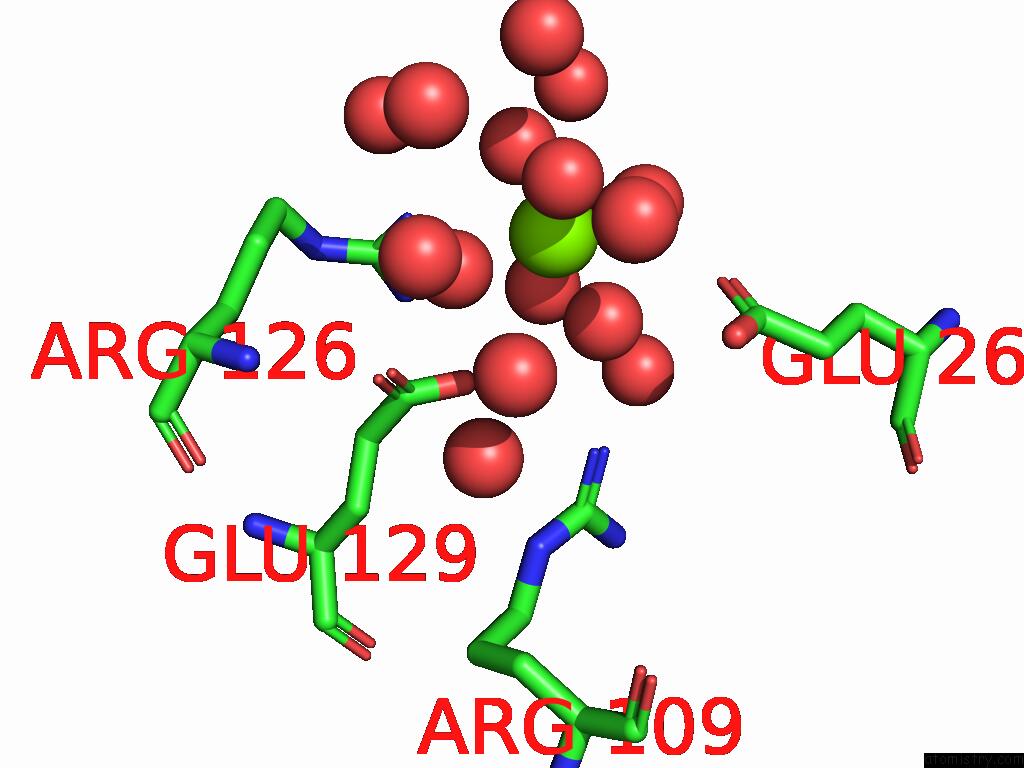
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
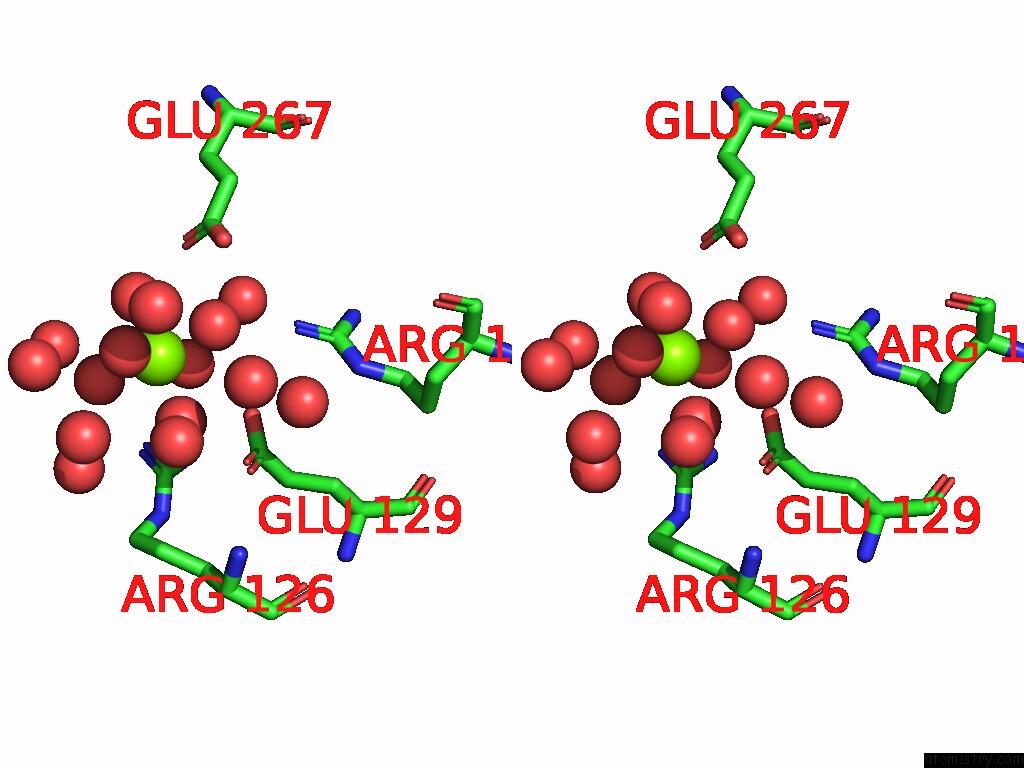
Magnesium binding site 6 out of 12 in 9ibe
Go back to
Magnesium binding site 6 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
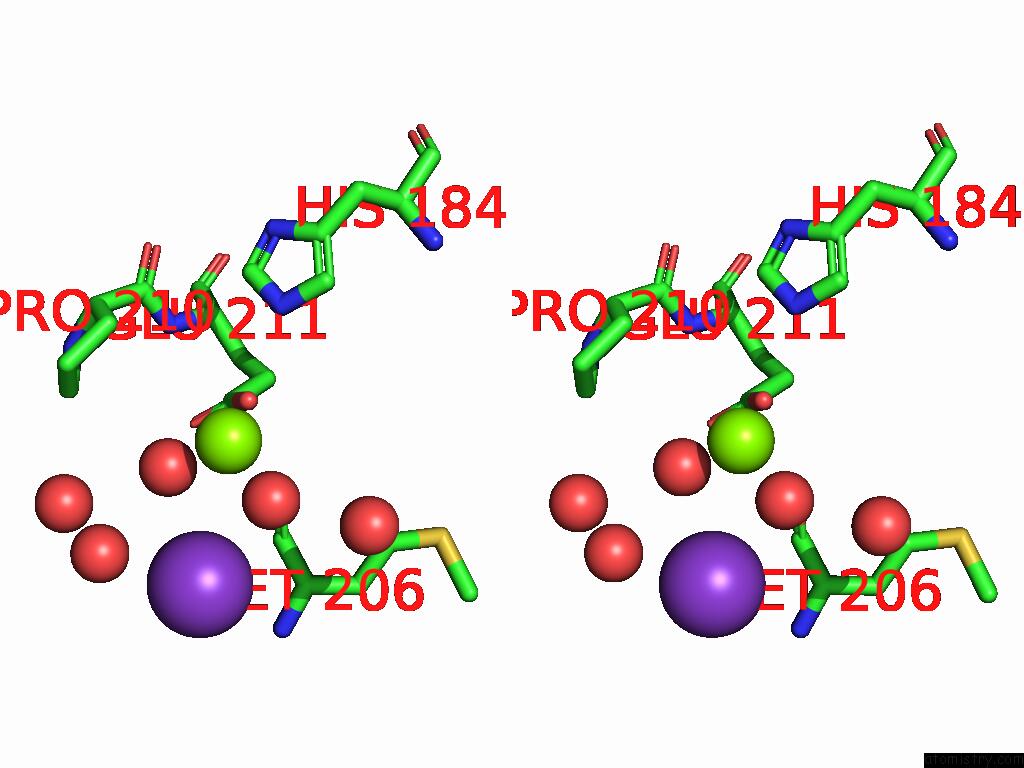
Magnesium binding site 7 out of 12 in 9ibe
Go back to
Magnesium binding site 7 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
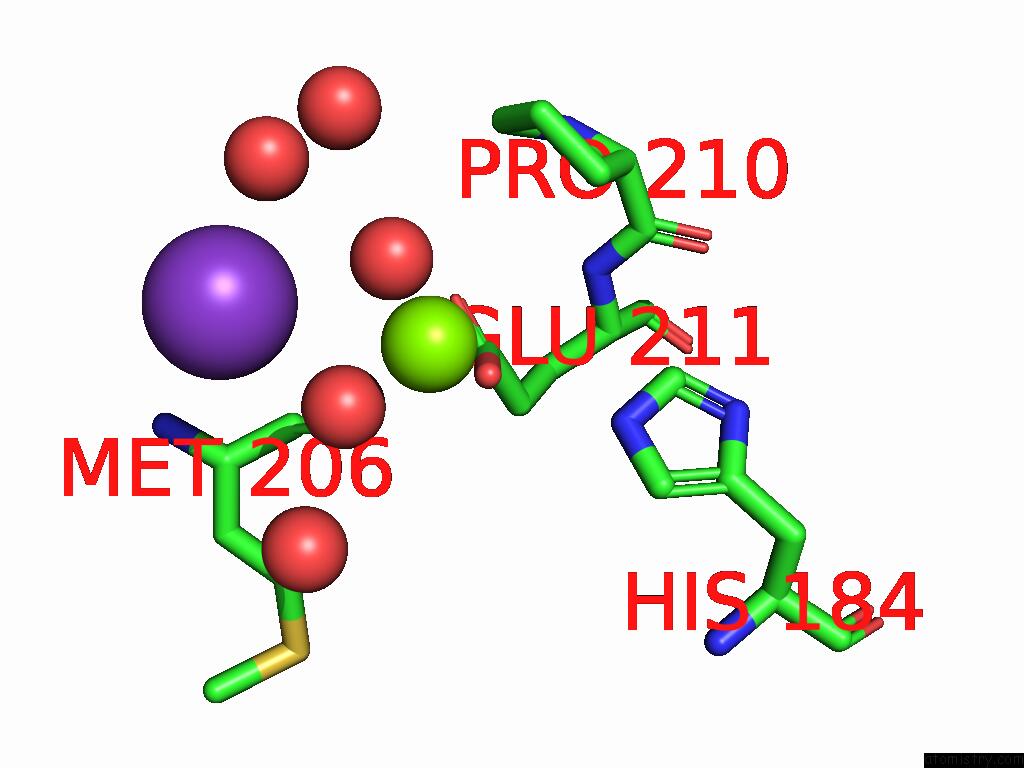
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
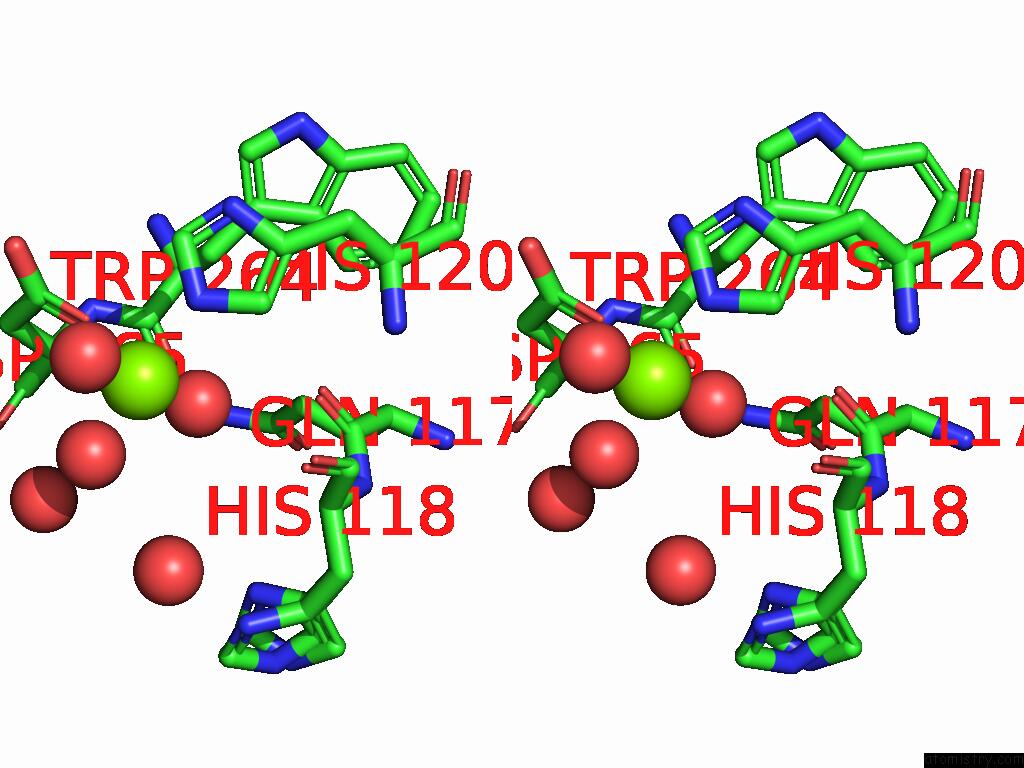
Magnesium binding site 8 out of 12 in 9ibe
Go back to
Magnesium binding site 8 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
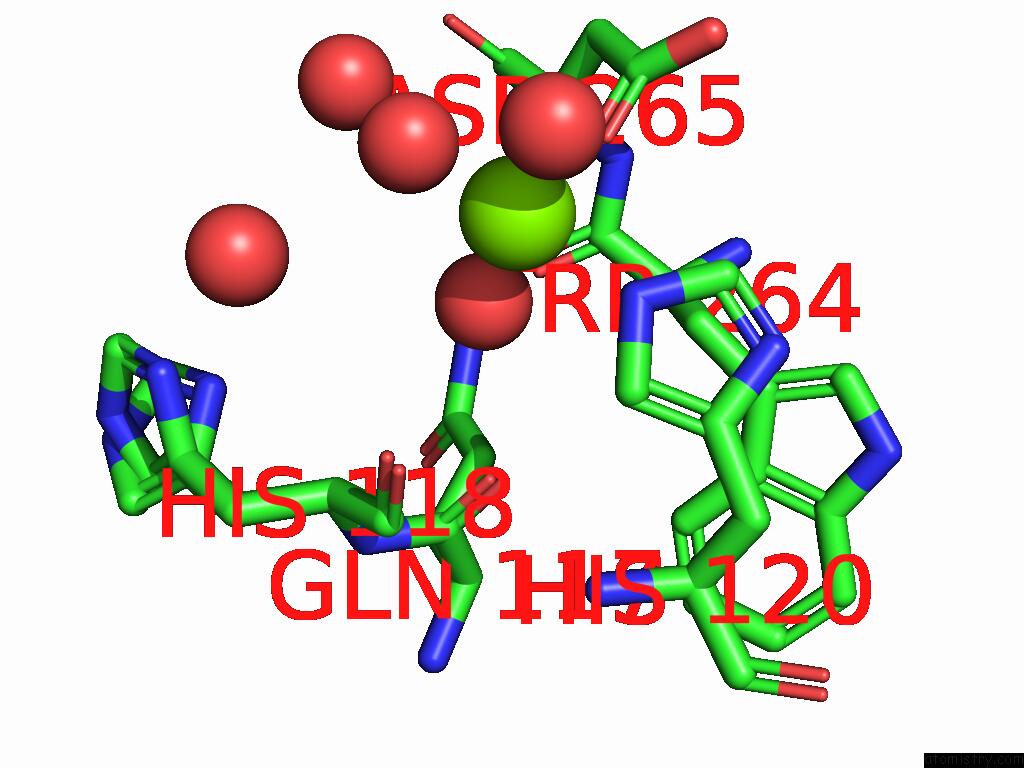
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
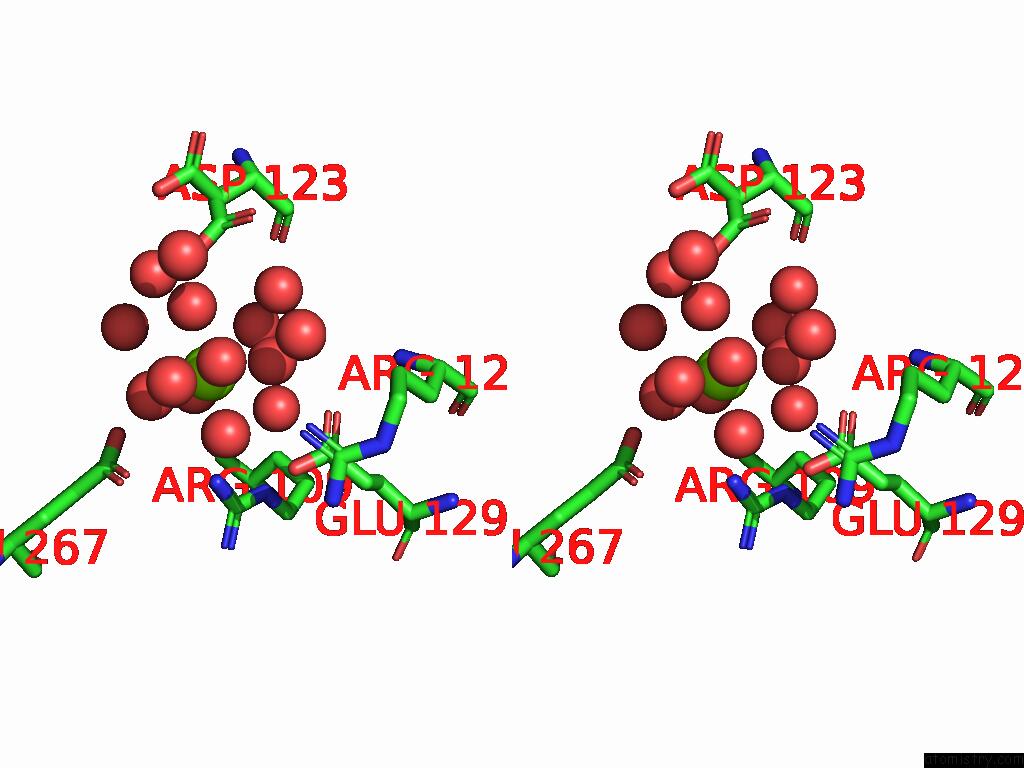
Magnesium binding site 9 out of 12 in 9ibe
Go back to
Magnesium binding site 9 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
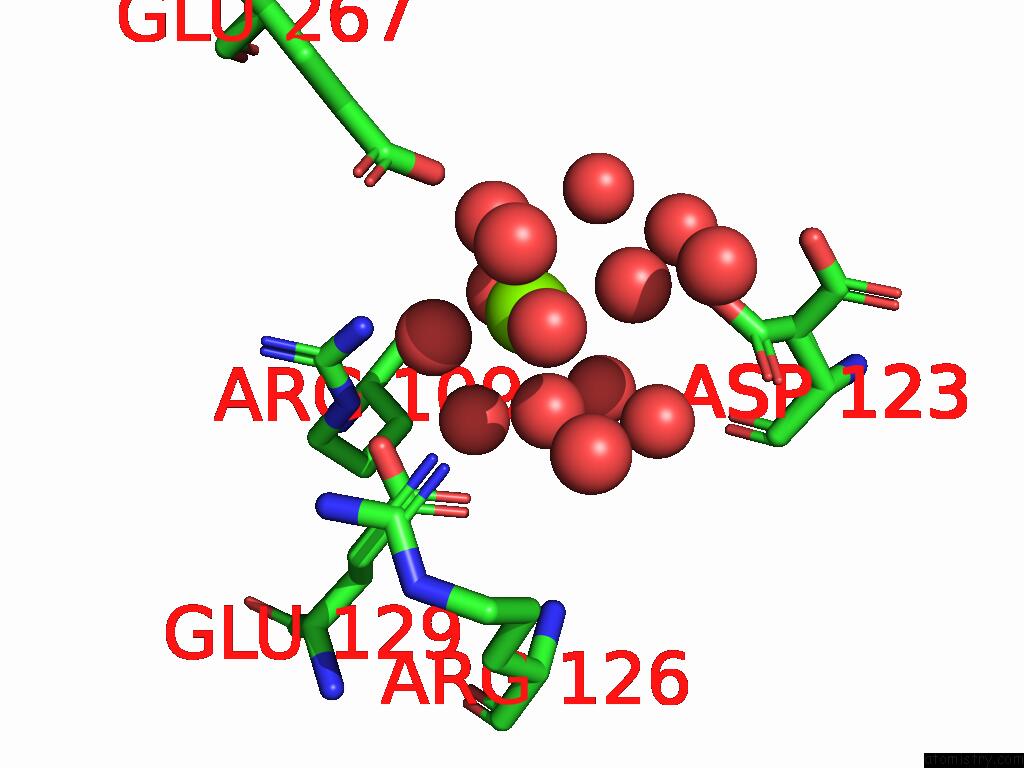
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
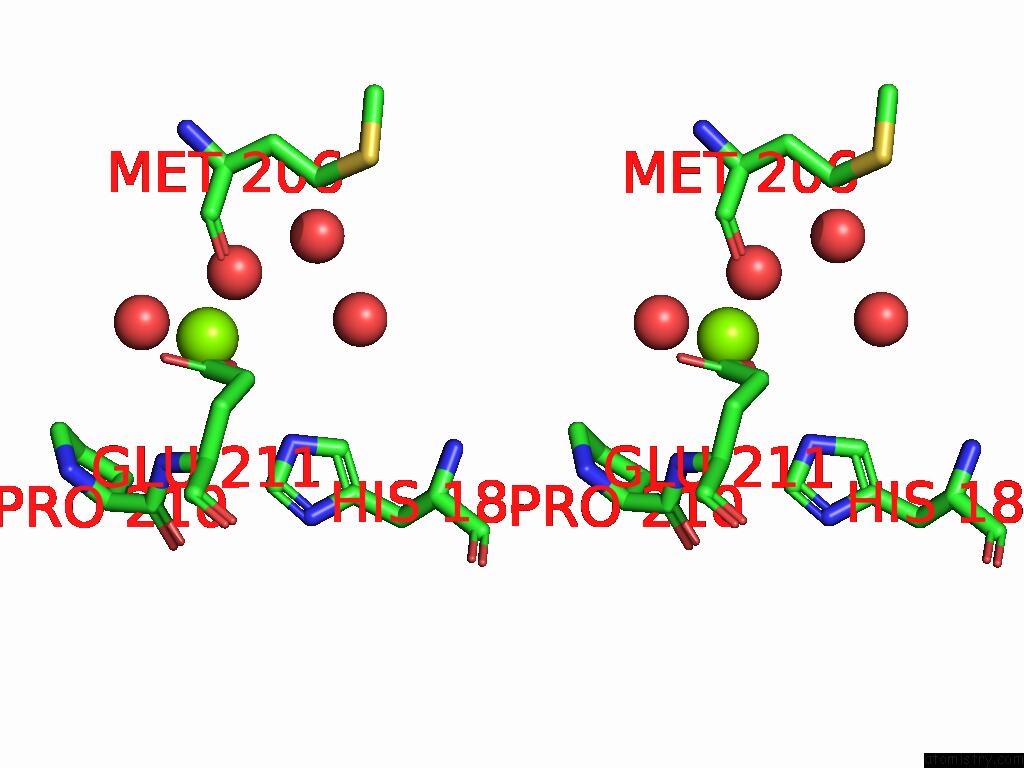
Magnesium binding site 10 out of 12 in 9ibe
Go back to
Magnesium binding site 10 out
of 12 in the D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride
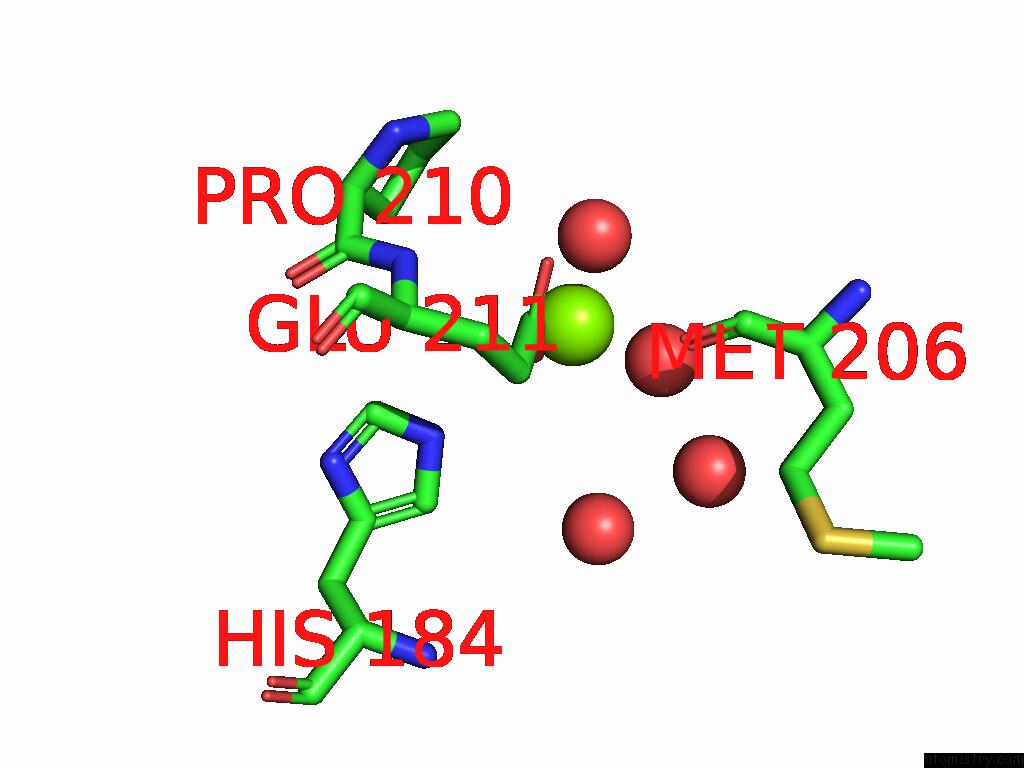
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 10 of D-2-Hydroxyacid Dehydrogenase (D2HDH) From Haloferax Mediterranei in Complex with Potassium, 2-Ketohexanoic Acid, Nadp+ and Chloride within 5.0Å range:
|
Reference:
J.Domenech,
N.Pramanpol,
C.Bisson,
S.E.Sedelnikova,
J.R.Barrett,
A.A.A.B.Dakhil,
V.Mykhaylyk,
A.S.Abdelhameed,
S.E.Harding,
D.W.Rice,
P.J.Baker,
J.Ferrer.
Potassium Binding By Carbonyl Clusters, Halophilic Adaptation and Catalysis of Haloferax Mediterranei D-2-Hydroxyacid Dehydrogenase. Commun Biol V. 8 1170 2025.
ISSN: ESSN 2399-3642
PubMed: 40770045
DOI: 10.1038/S42003-025-08587-7
Page generated: Tue Aug 26 21:11:39 2025
ISSN: ESSN 2399-3642
PubMed: 40770045
DOI: 10.1038/S42003-025-08587-7
Last articles
Zn in 9QM9Zn in 9S44
Zn in 9OFE
Zn in 9OFC
Zn in 9OFD
Zn in 9OF1
Zn in 9OFB
Zn in 9N0J
Zn in 9M5X
Zn in 9LGI