Magnesium »
PDB 9nf0-9qof »
9otp »
Magnesium in PDB 9otp: Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Enzymatic activity of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
All present enzymatic activity of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions:
2.3.1.225; 6.3.1.2;
2.3.1.225; 6.3.1.2;
Magnesium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20;Binding sites:
The binding sites of Magnesium atom in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions (pdb code 9otp). This binding sites where shown within 5.0 Angstroms radius around Magnesium atom.In total 20 binding sites of Magnesium where determined in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions, PDB code: 9otp:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Magnesium binding site 1 out of 20 in 9otp
Go back to
Magnesium binding site 1 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
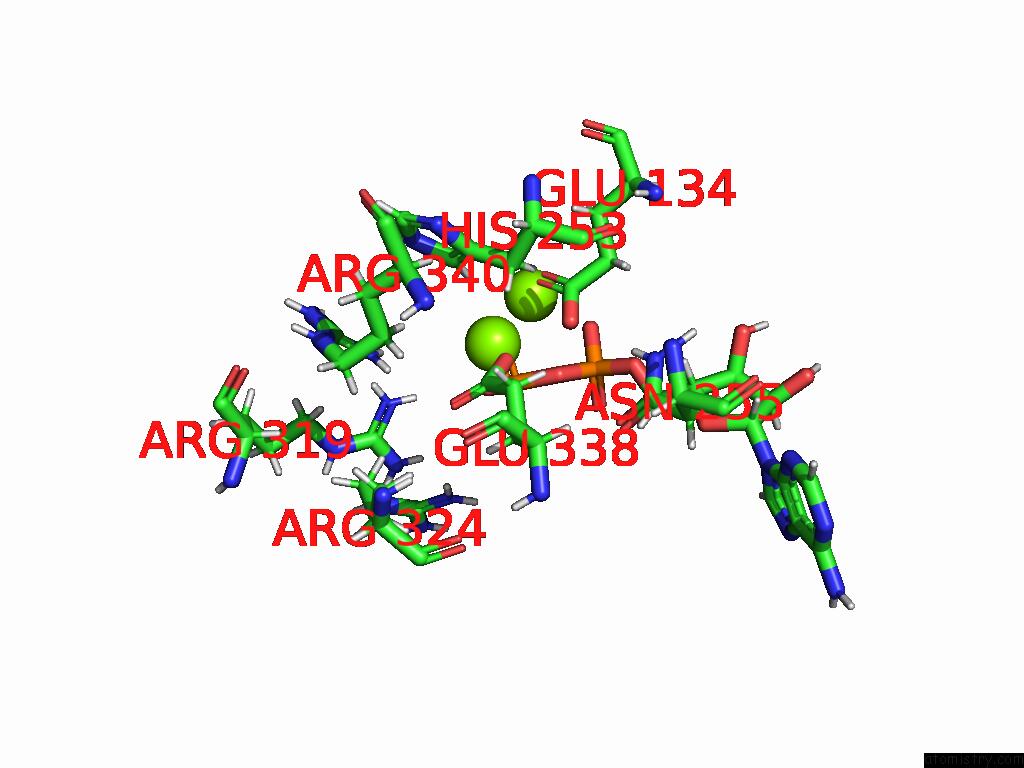
Mono view
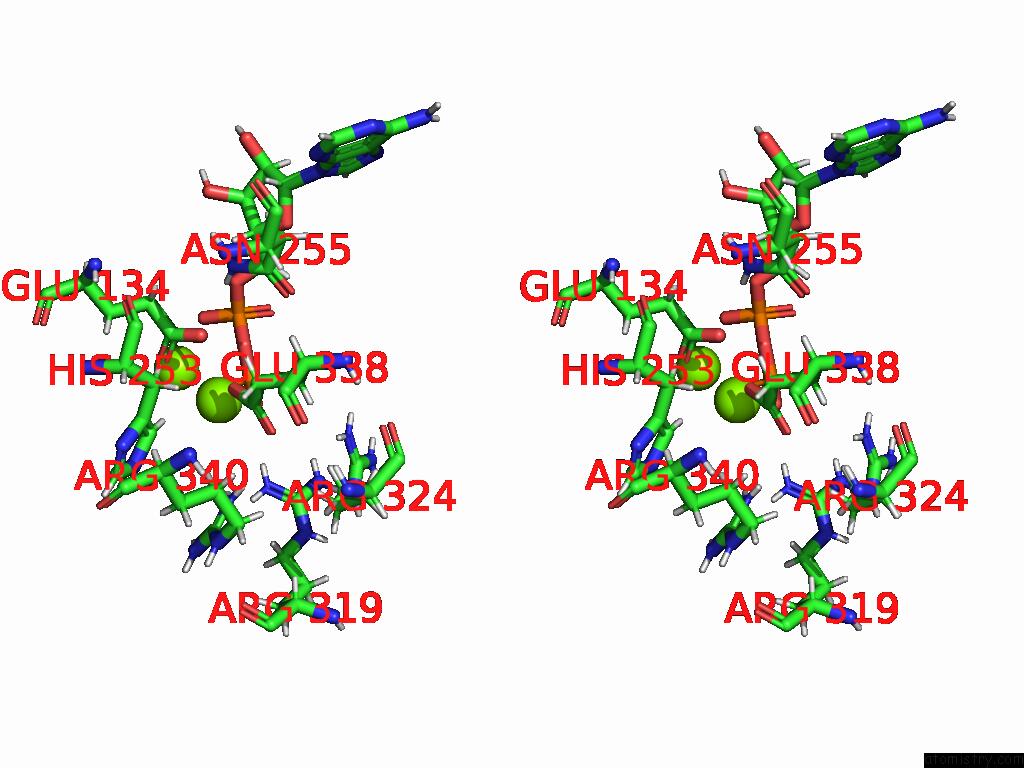
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
Magnesium binding site 2 out of 20 in 9otp
Go back to
Magnesium binding site 2 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
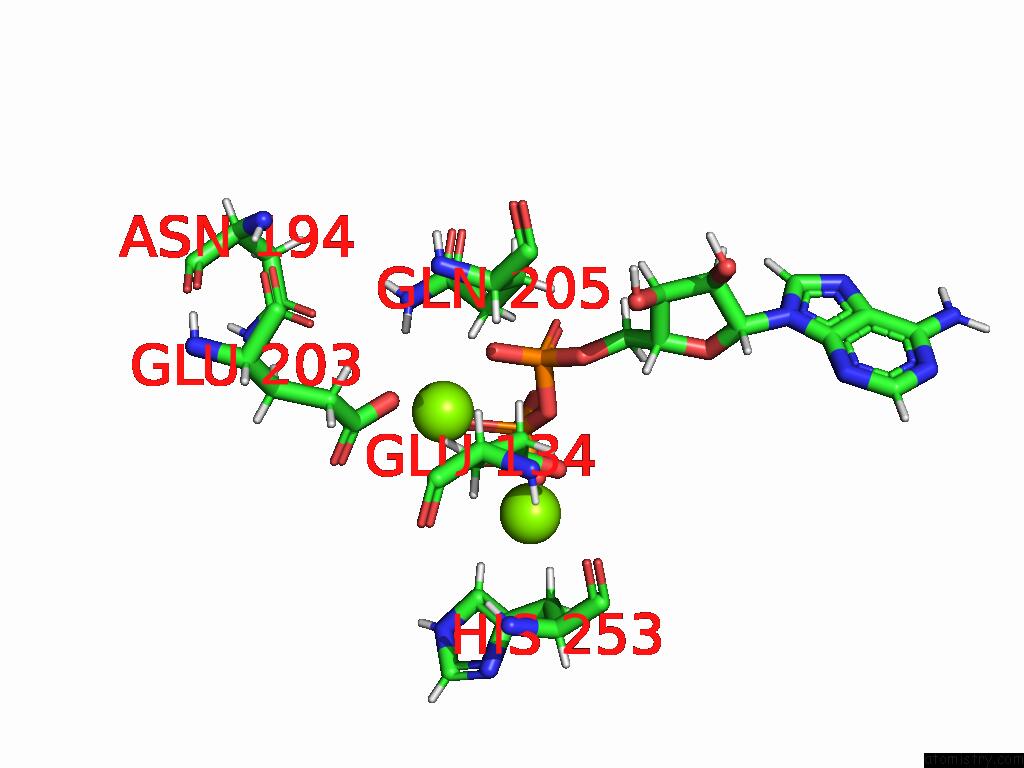
Mono view
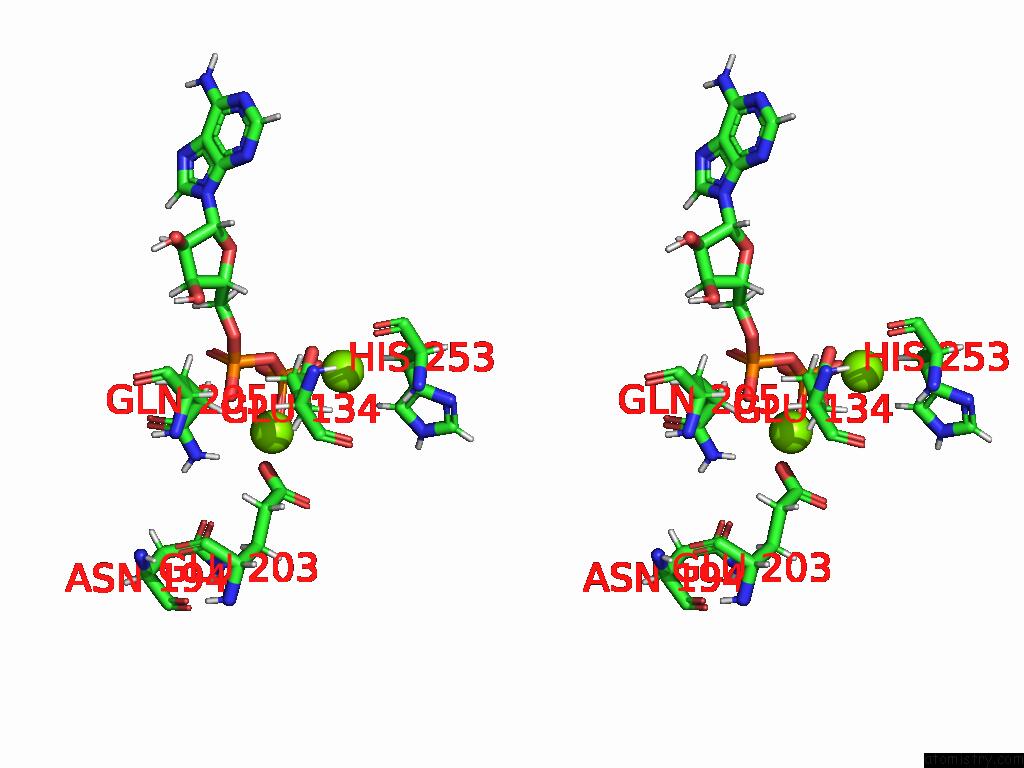
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
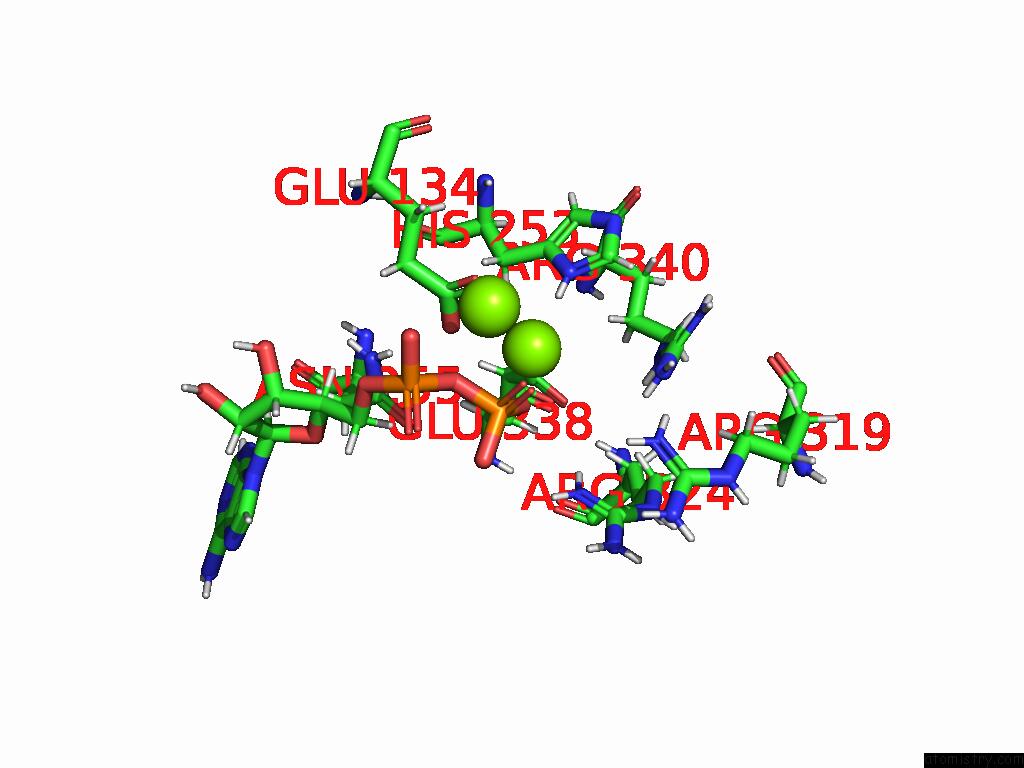
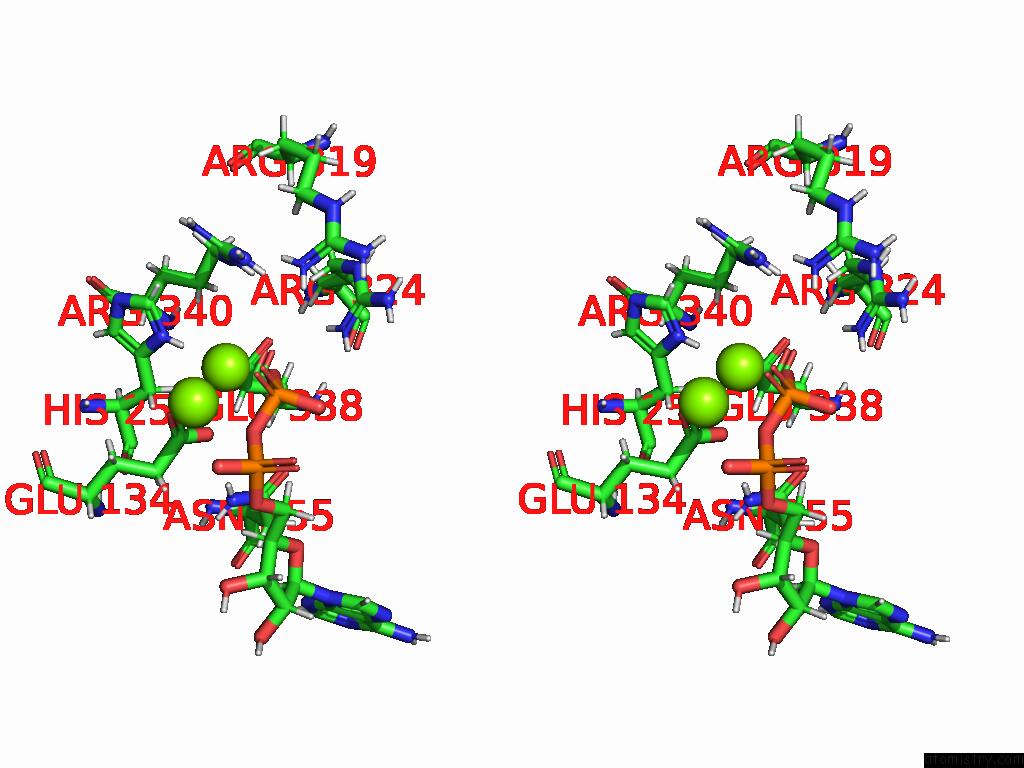
Magnesium binding site 3 out of 20 in 9otp
Go back to
Magnesium binding site 3 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
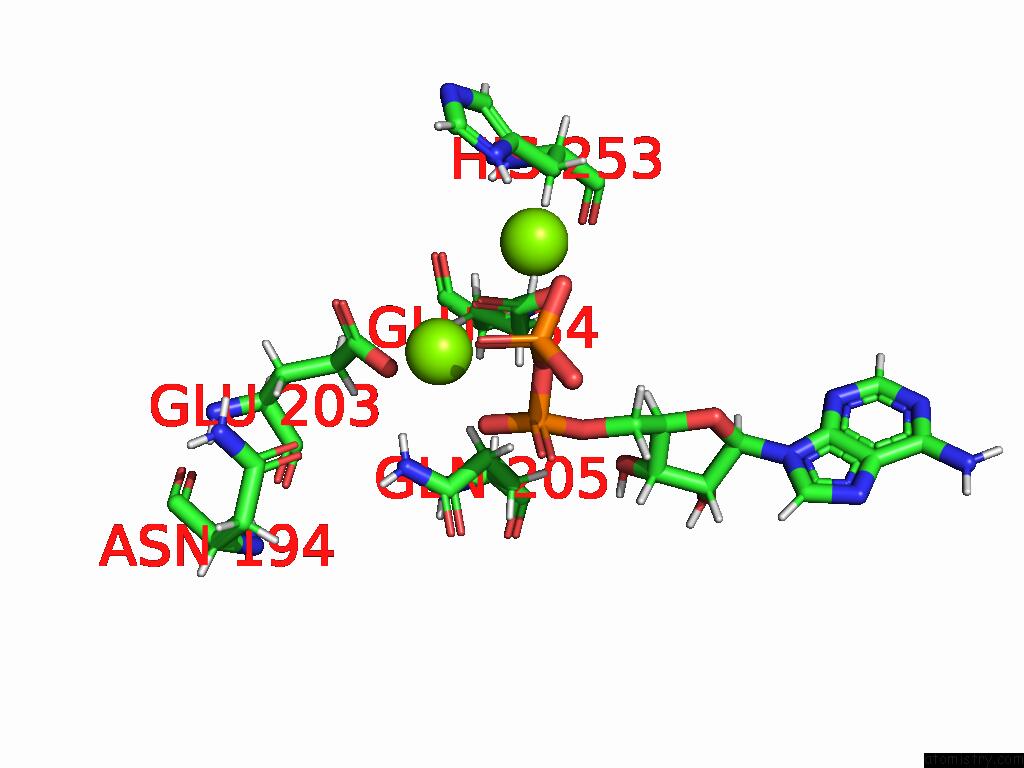
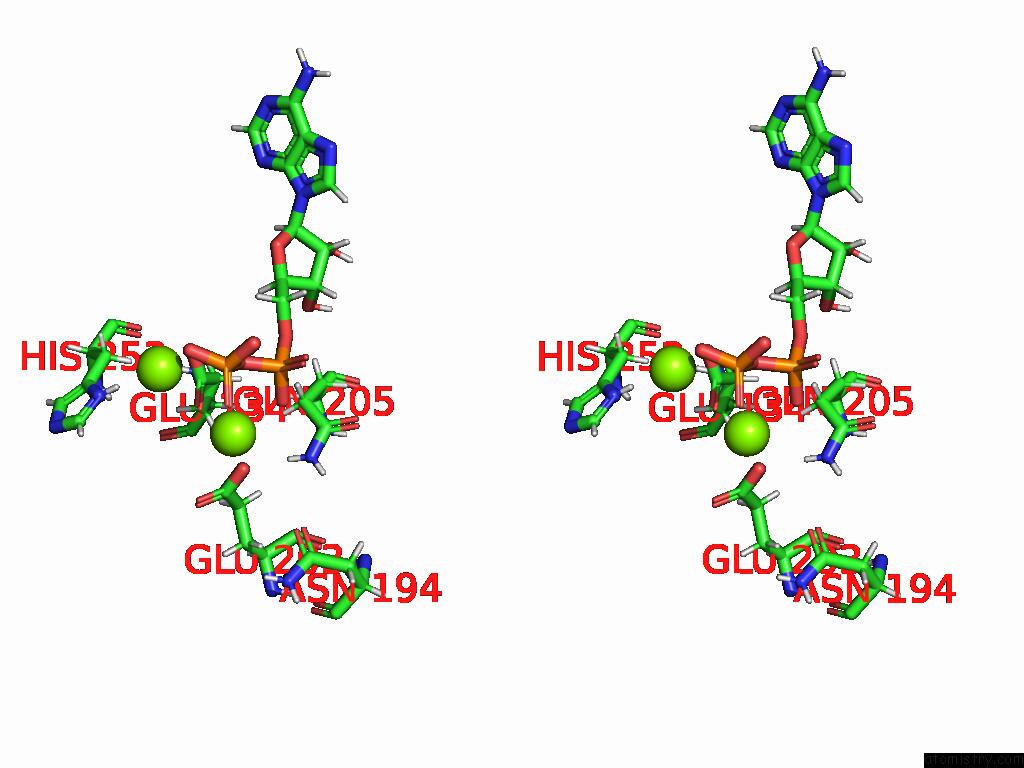
Magnesium binding site 4 out of 20 in 9otp
Go back to
Magnesium binding site 4 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
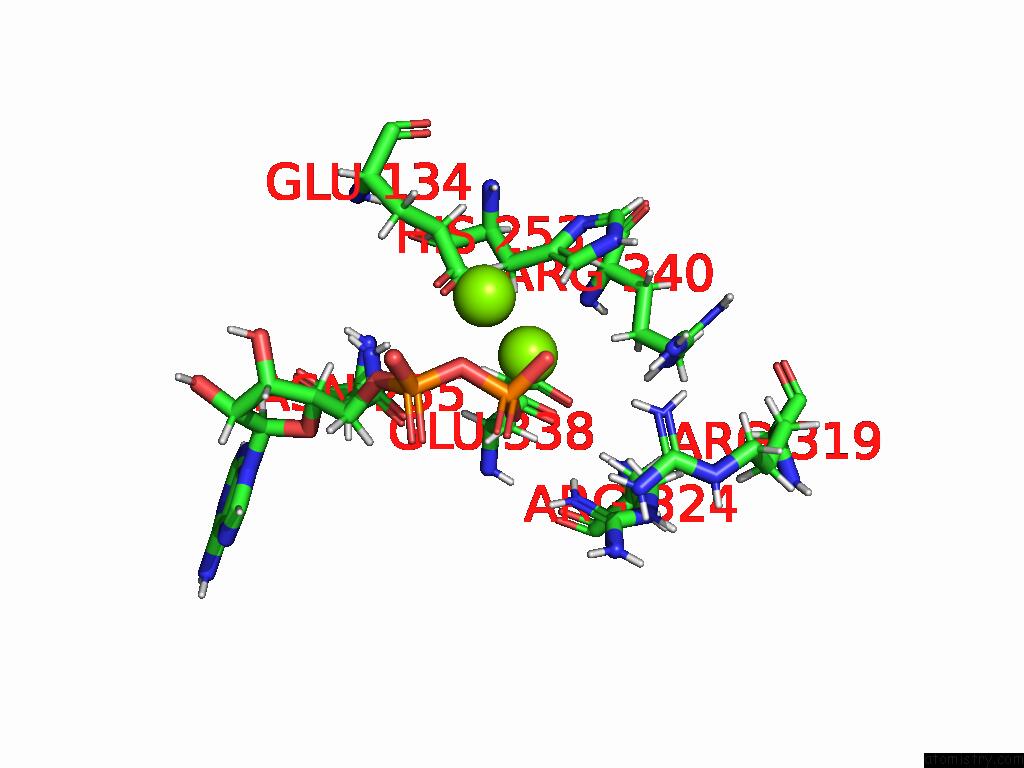
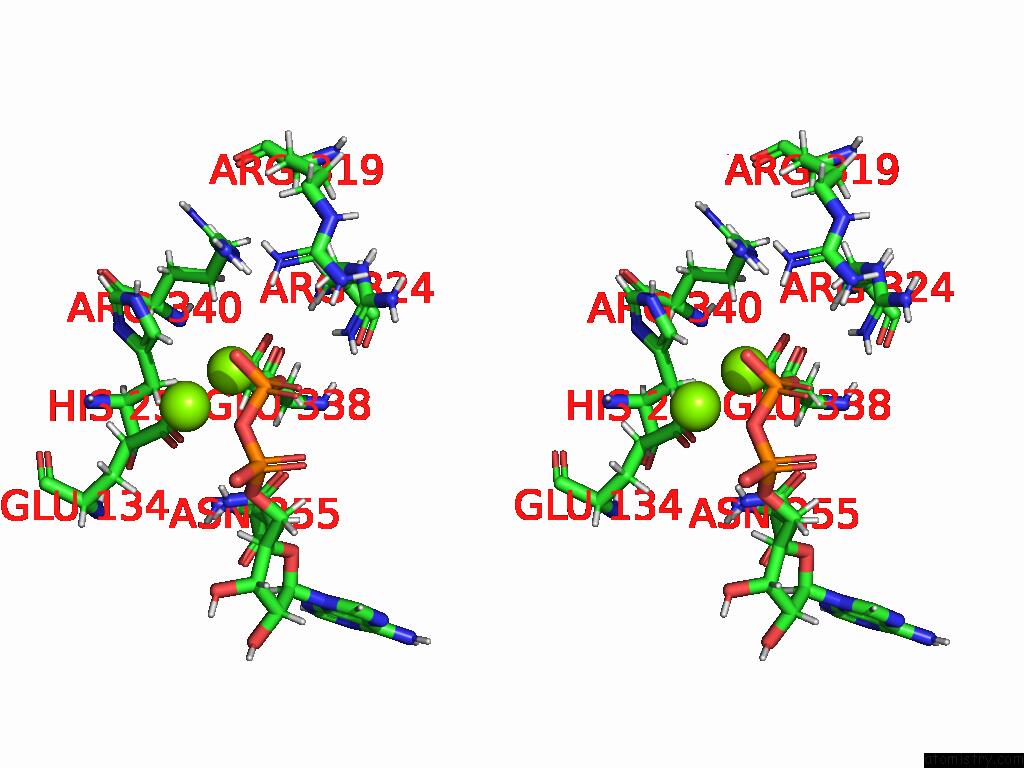
Magnesium binding site 5 out of 20 in 9otp
Go back to
Magnesium binding site 5 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
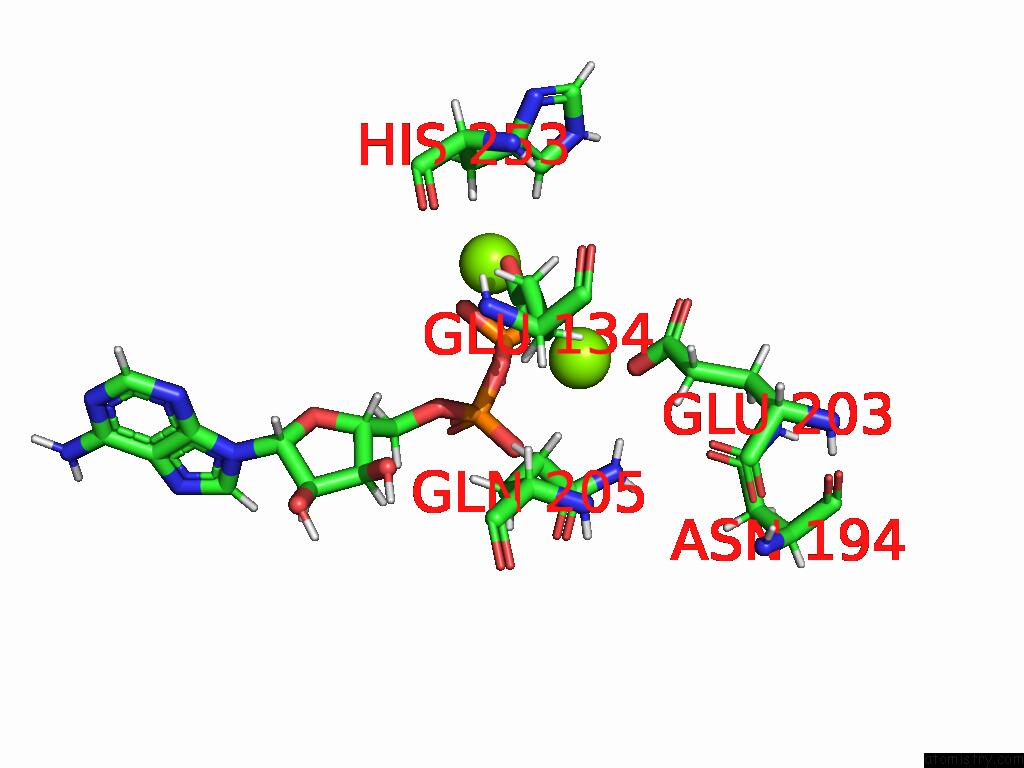
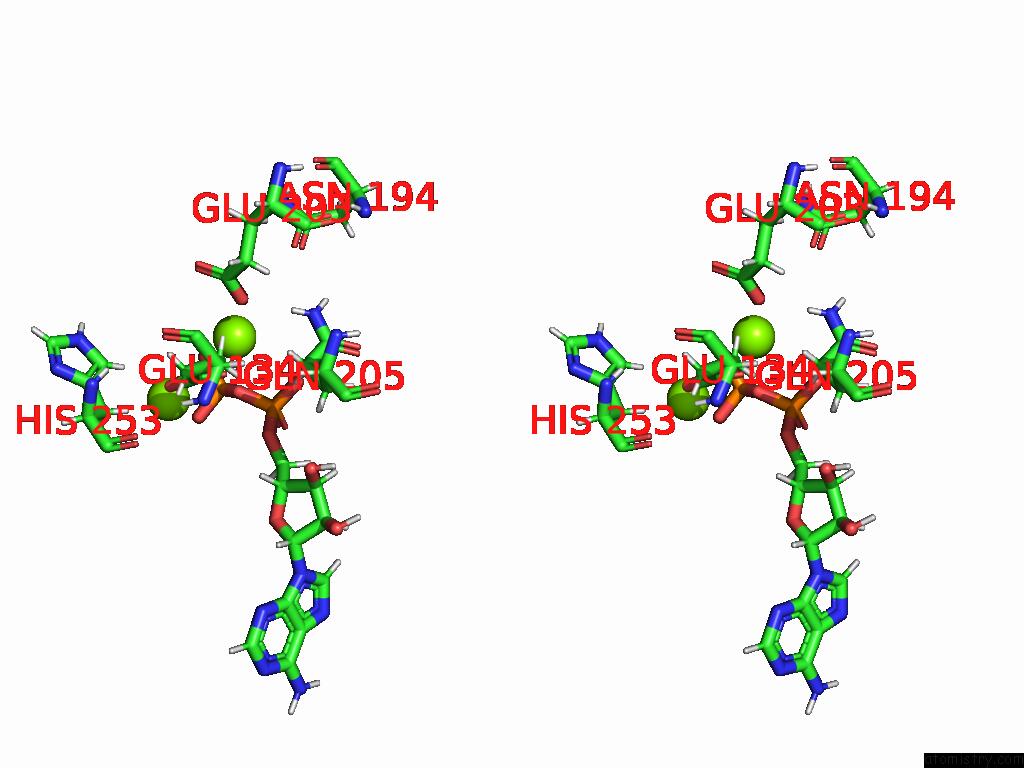
Magnesium binding site 6 out of 20 in 9otp
Go back to
Magnesium binding site 6 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
Magnesium binding site 7 out of 20 in 9otp
Go back to
Magnesium binding site 7 out
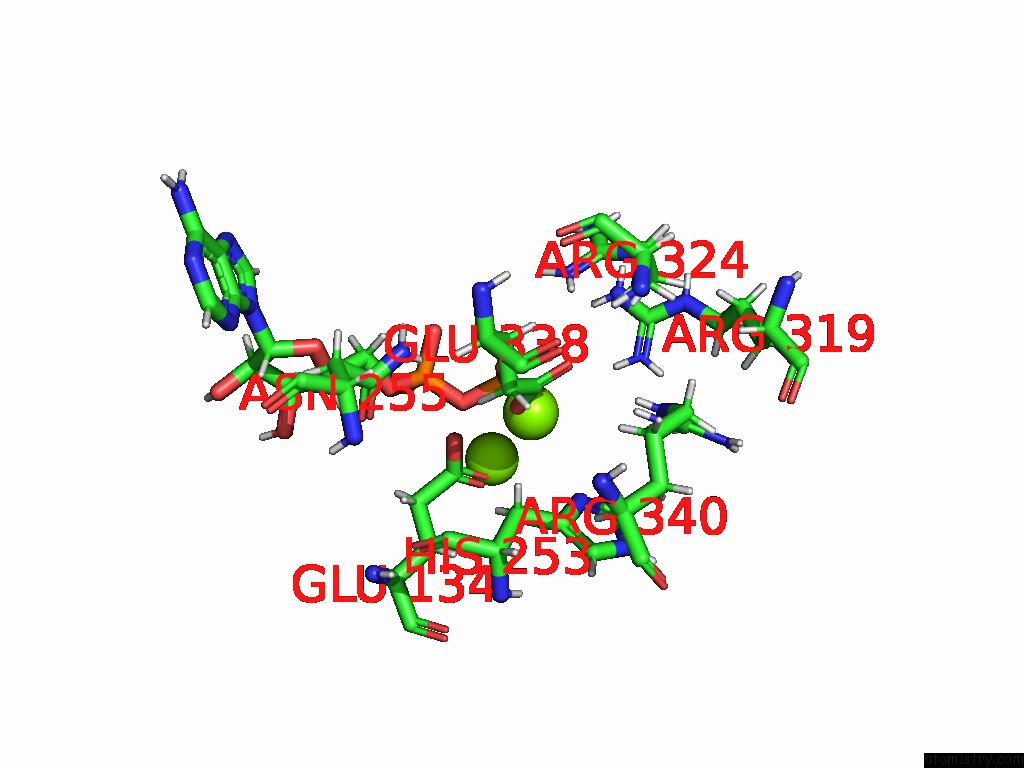
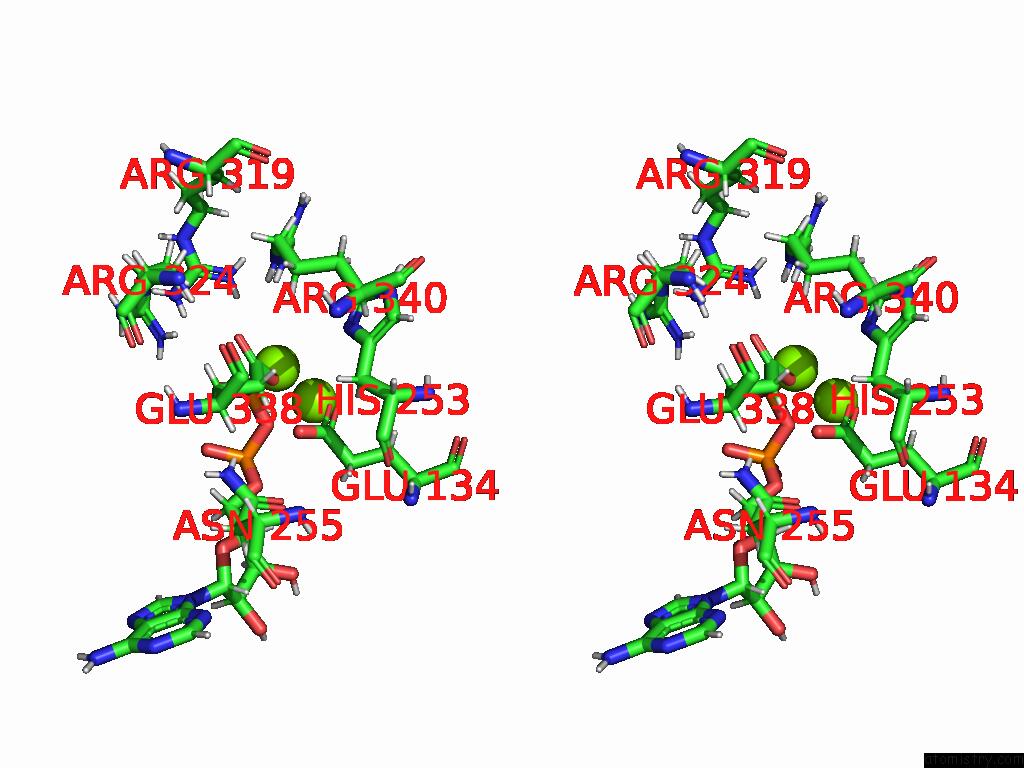
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
Magnesium binding site 8 out of 20 in 9otp
Go back to
Magnesium binding site 8 out
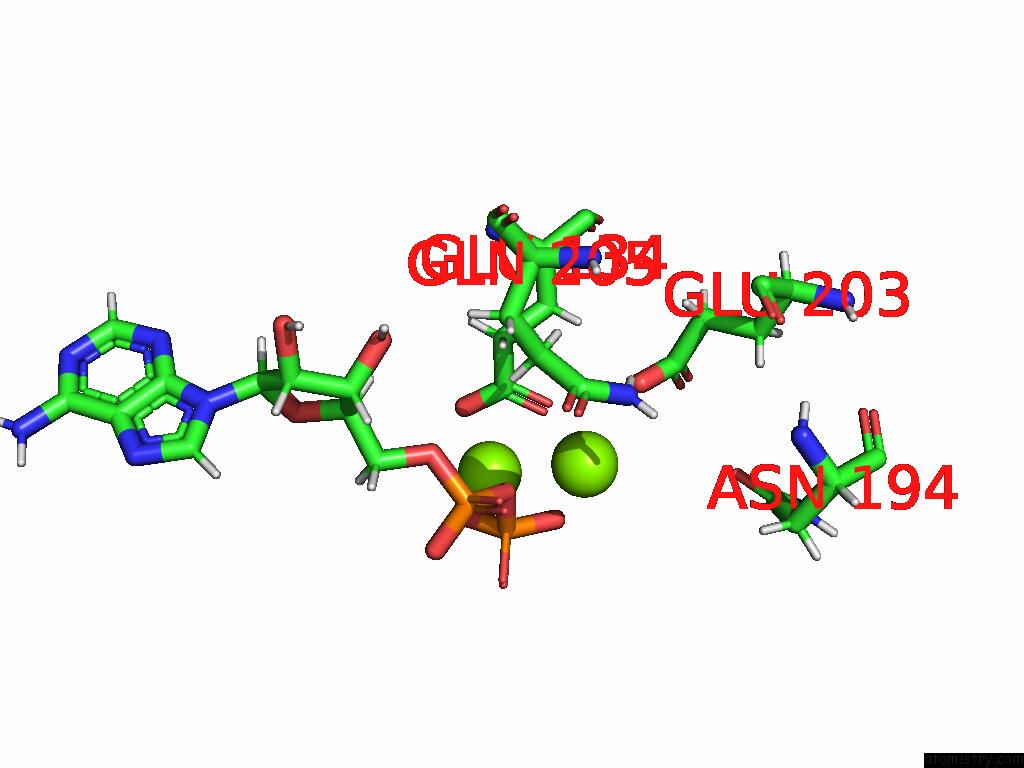
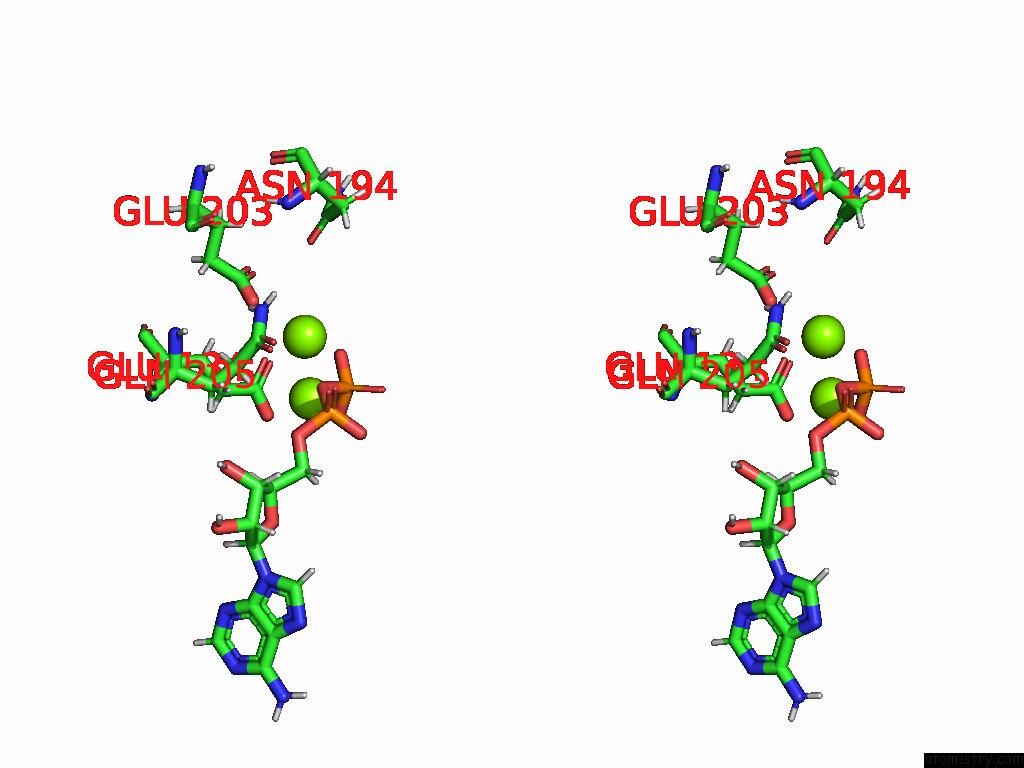
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
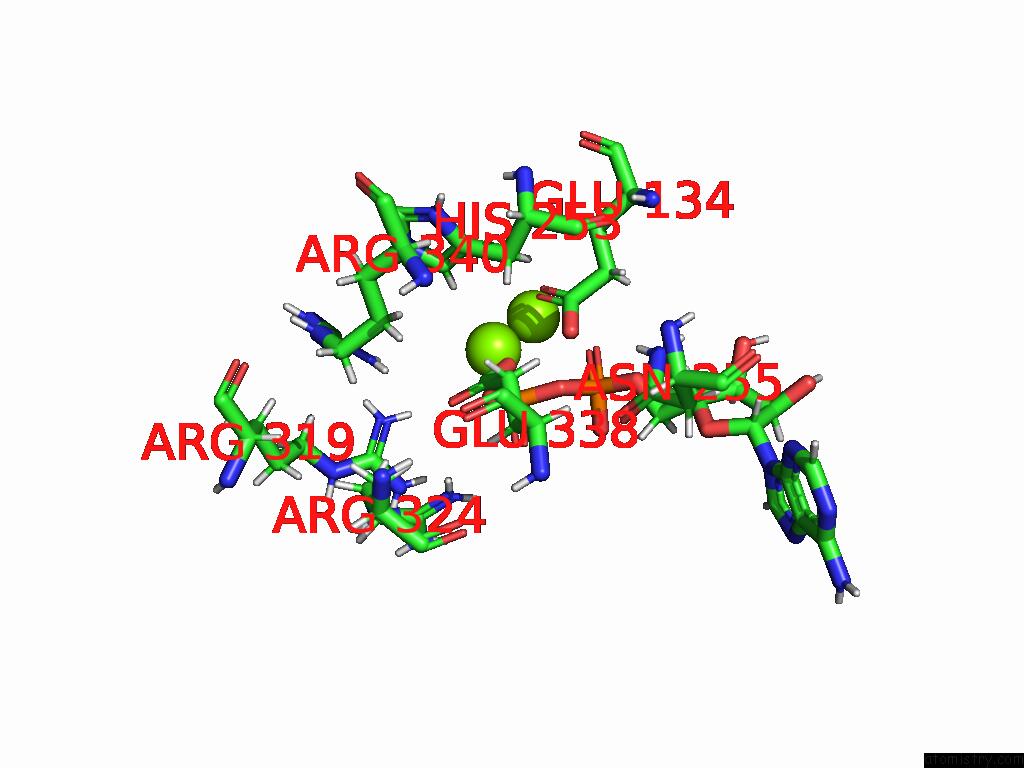
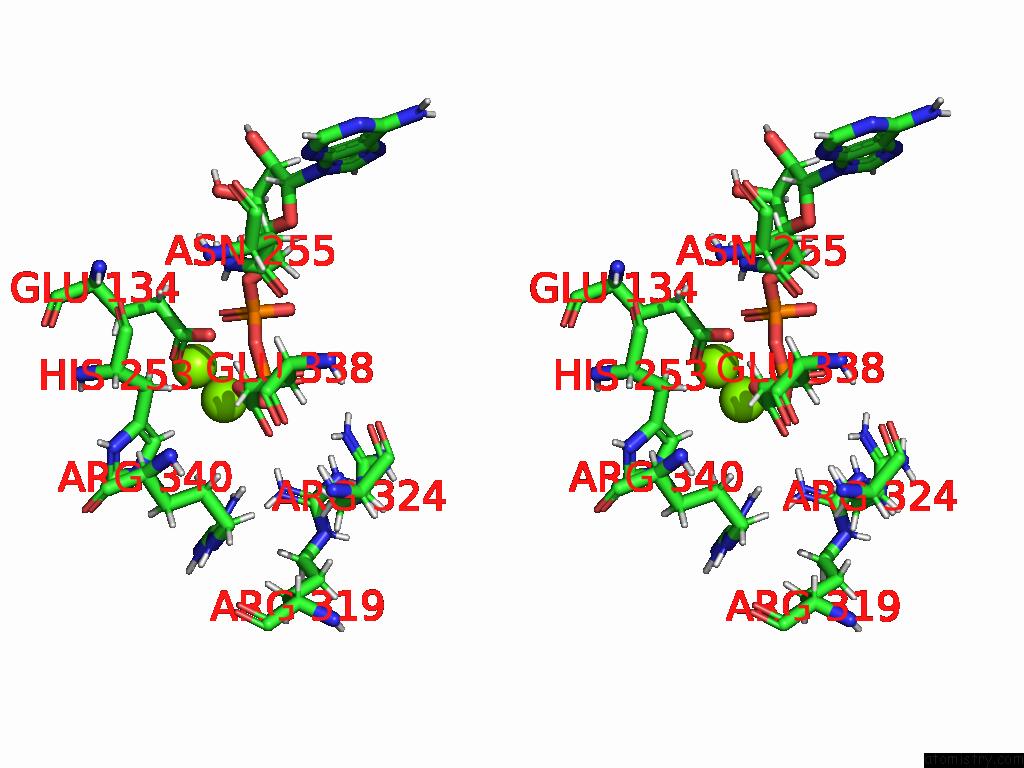
Magnesium binding site 9 out of 20 in 9otp
Go back to
Magnesium binding site 9 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
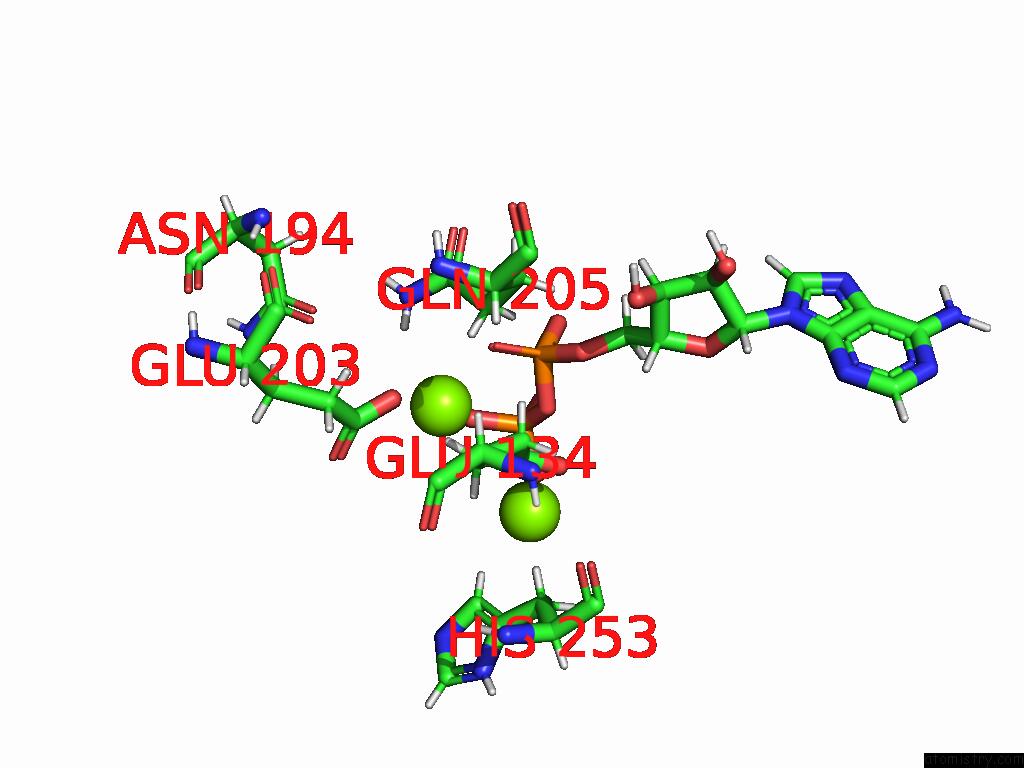
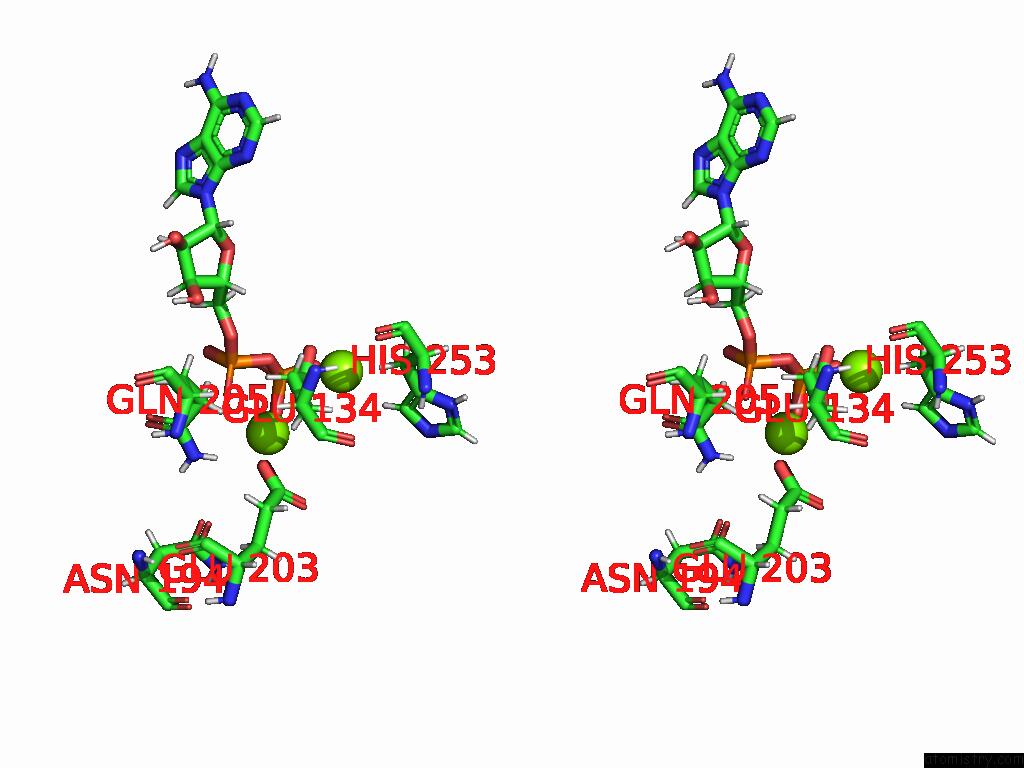
Magnesium binding site 10 out of 20 in 9otp
Go back to
Magnesium binding site 10 out
of 20 in the Human Glutamine Synthetase R298A Decamer Under Turnover Conditions
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 10 of Human Glutamine Synthetase R298A Decamer Under Turnover Conditions within 5.0Å range:
|
Reference:
E.R.Greene,
R.S.Muniz,
H.Yamamura,
S.Hoff,
P.Bajaj,
D.J.Lee,
E.M.Thompson,
A.Arada,
M.Bonomi,
J.M.Kollman,
J.S.Fraser.
Product-Stabilized Filamentation By Human Glutamine Synthetase Allosterically Tunes Metabolic Activity To Be Published.
Page generated: Sat Aug 16 07:06:55 2025
Last articles
Na in 3TVBNa in 3TXJ
Na in 3TXK
Na in 3TXI
Na in 3TXH
Na in 3TXG
Na in 3TXD
Na in 3TXF
Na in 3TXE
Na in 3TXB