Magnesium »
PDB 1k9w-1kk3 »
1kh3 »
Magnesium in PDB 1kh3: Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
Enzymatic activity of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
All present enzymatic activity of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor:
6.3.4.5;
6.3.4.5;
Protein crystallography data
The structure of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor, PDB code: 1kh3
was solved by
M.Goto,
K.Hirotsu,
I.Miyahara,
Riken Structuralgenomics/Proteomics Initiative (Rsgi),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 2.15 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 229.100, 229.100, 160.600, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 22.6 / 25.6 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
(pdb code 1kh3). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor, PDB code: 1kh3:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor, PDB code: 1kh3:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 1kh3
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
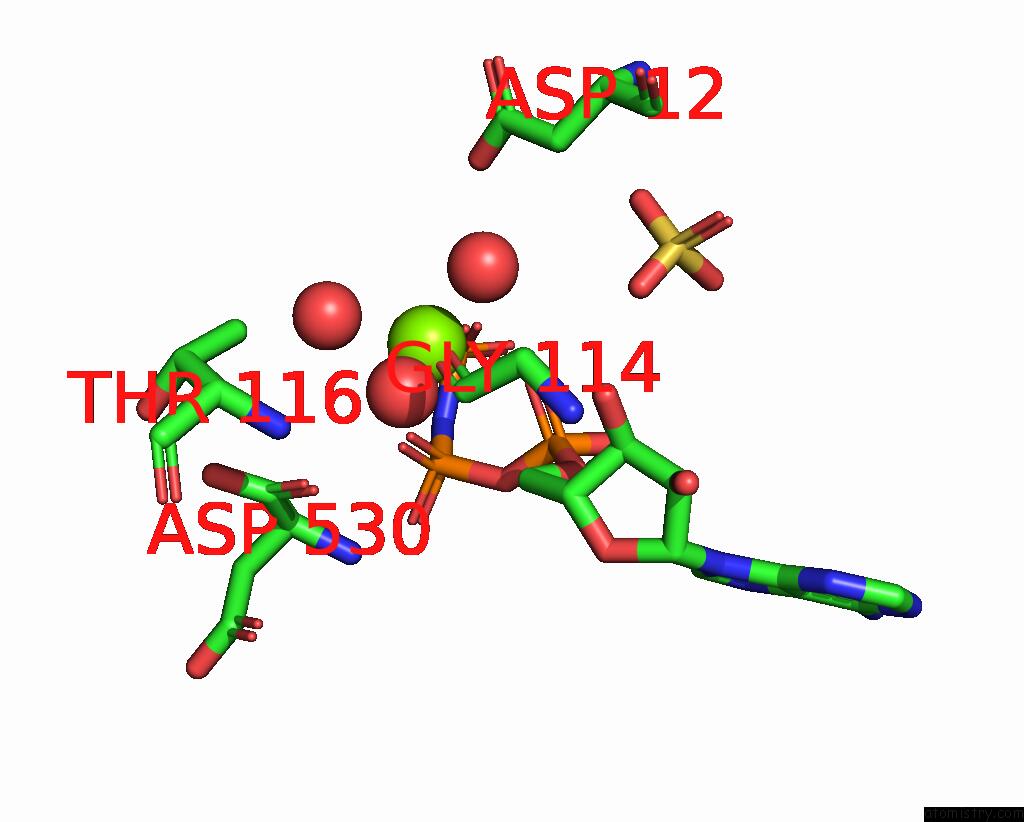
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor within 5.0Å range:
|
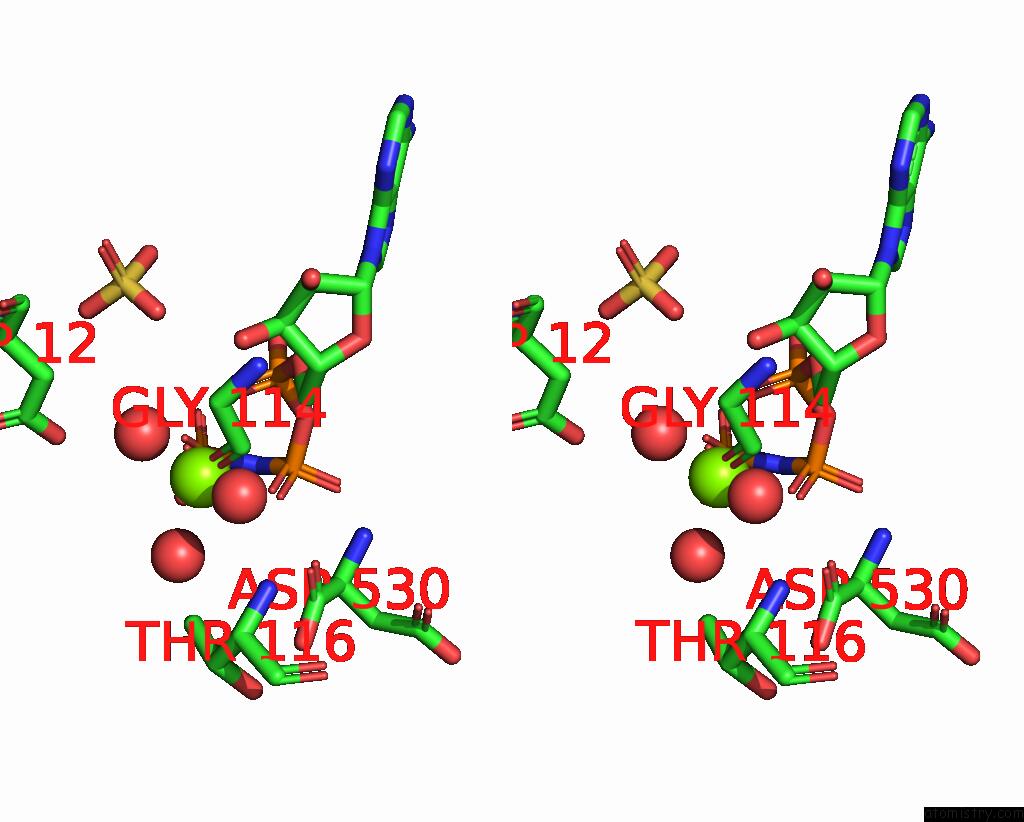
Magnesium binding site 2 out of 4 in 1kh3
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor within 5.0Å range:
|
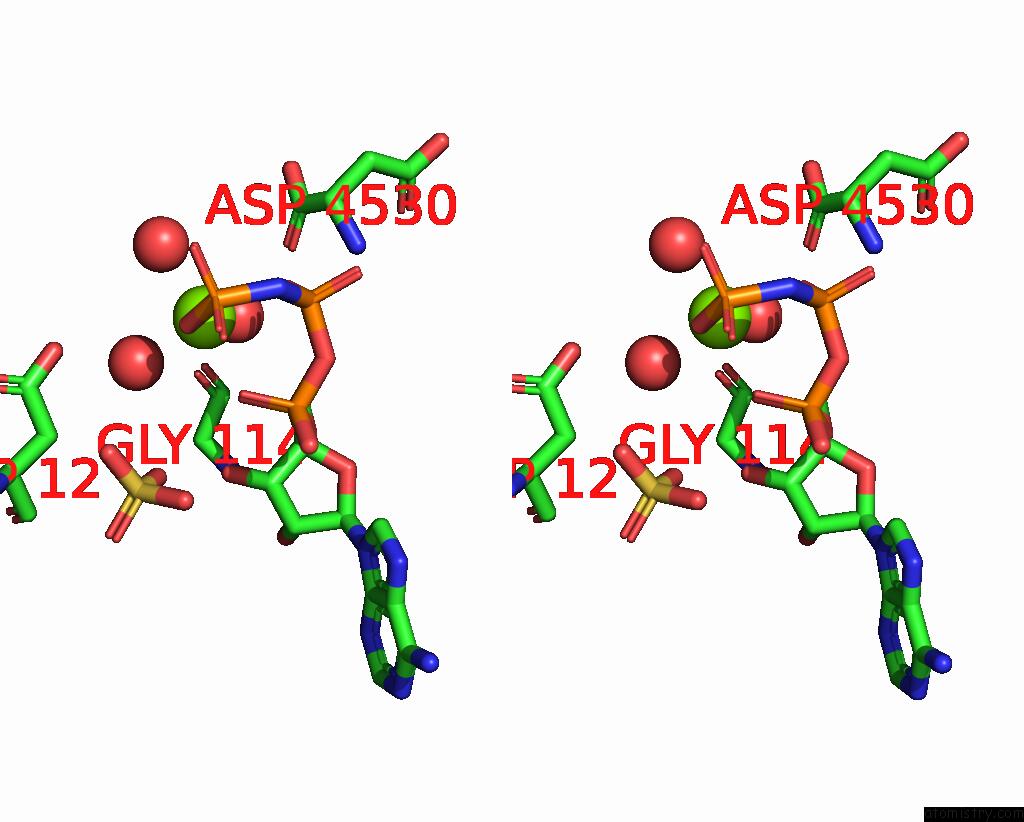
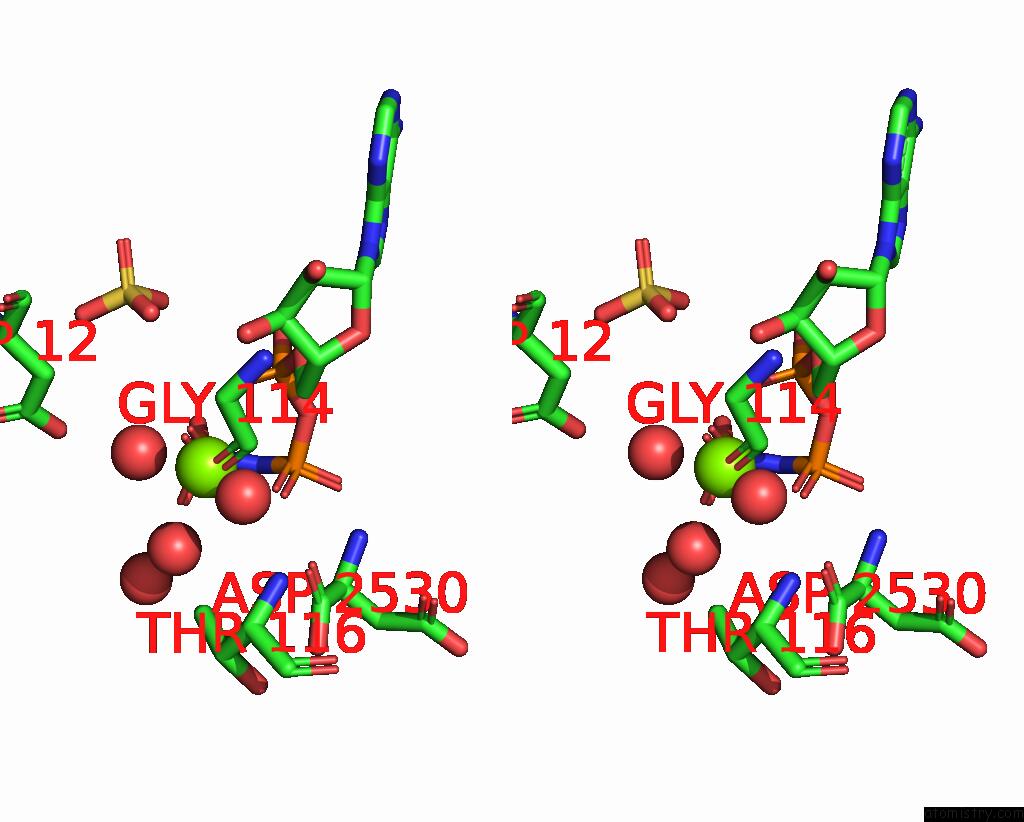
Magnesium binding site 3 out of 4 in 1kh3
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor within 5.0Å range:
|
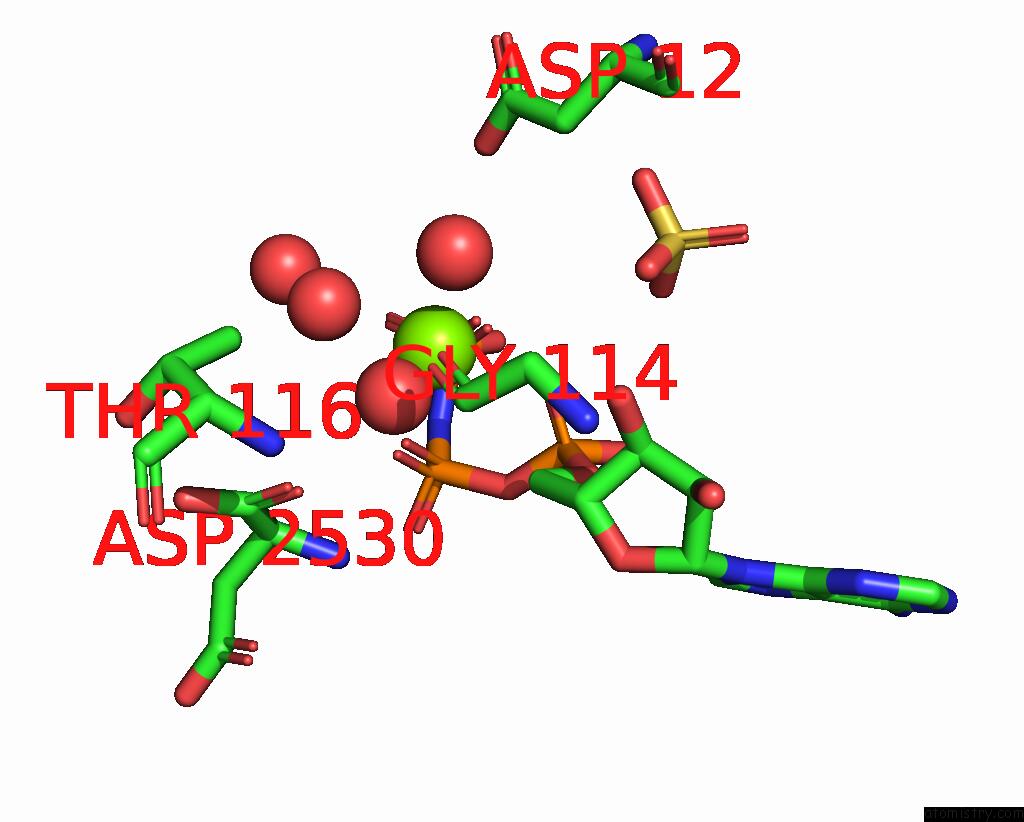
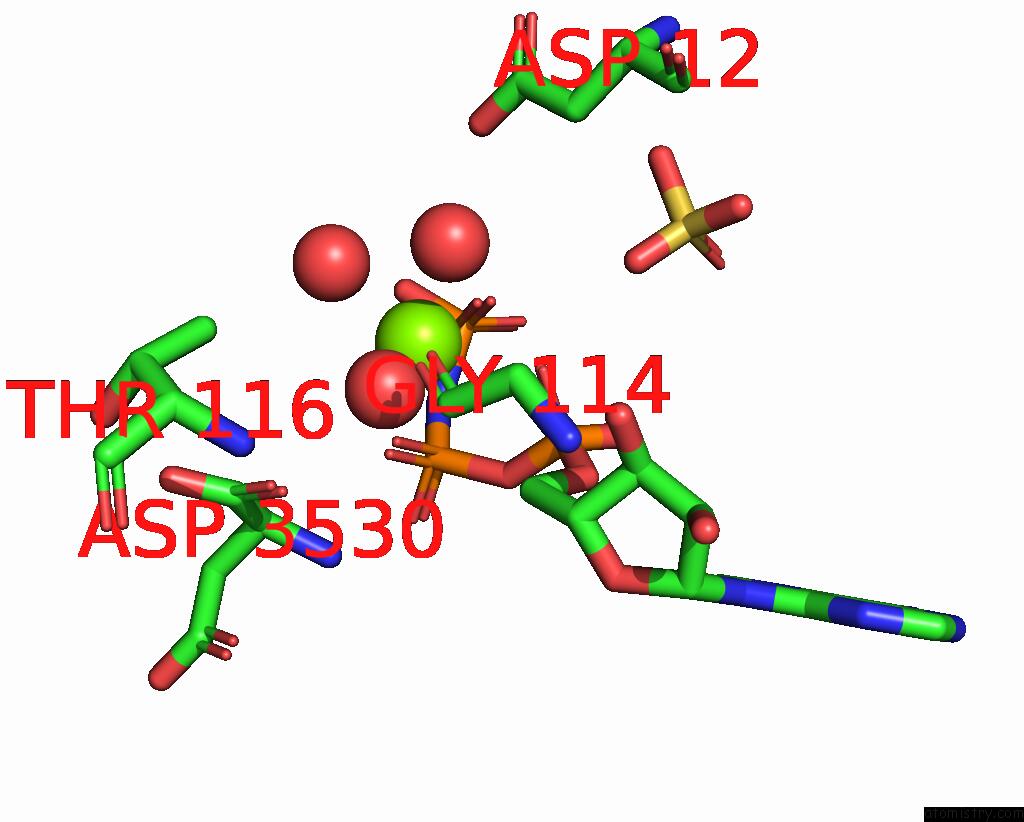
Magnesium binding site 4 out of 4 in 1kh3
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Thermus Thermophilus HB8 Argininosuccinate Synthetase in Complex with Inhibitor within 5.0Å range:
|
Reference:
M.Goto,
R.Omi,
I.Miyahara,
M.Sugahara,
K.Hirotsu.
Structures of Argininosuccinate Synthetase in Enzyme-Atp Substrates and Enzyme-Amp Product Forms: Stereochemistry of the Catalytic Reaction J.Biol.Chem. V. 278 22964 2003.
ISSN: ISSN 0021-9258
PubMed: 12684518
DOI: 10.1074/JBC.M213198200
Page generated: Sun Aug 10 00:08:57 2025
ISSN: ISSN 0021-9258
PubMed: 12684518
DOI: 10.1074/JBC.M213198200
Last articles
Mg in 1VQ4Mg in 1VPA
Mg in 1VPE
Mg in 1VOM
Mg in 1VMA
Mg in 1VMK
Mg in 1VM9
Mg in 1VCR
Mg in 1VLB
Mg in 1VKP