Magnesium »
PDB 1lnz-1mez »
1lnz »
Magnesium in PDB 1lnz: Structure of the Obg Gtp-Binding Protein
Protein crystallography data
The structure of Structure of the Obg Gtp-Binding Protein, PDB code: 1lnz
was solved by
J.Buglino,
V.Shen,
P.Hakimian,
C.D.Lima,
S.K.Burley,
New Yorksgx Research Center For Structural Genomics (Nysgxrc),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.89 / 2.60 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.580, 105.006, 124.098, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.3 / 29.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the Obg Gtp-Binding Protein
(pdb code 1lnz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 7 binding sites of Magnesium where determined in the Structure of the Obg Gtp-Binding Protein, PDB code: 1lnz:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Magnesium where determined in the Structure of the Obg Gtp-Binding Protein, PDB code: 1lnz:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7;
Magnesium binding site 1 out of 7 in 1lnz
Go back to
Magnesium binding site 1 out
of 7 in the Structure of the Obg Gtp-Binding Protein
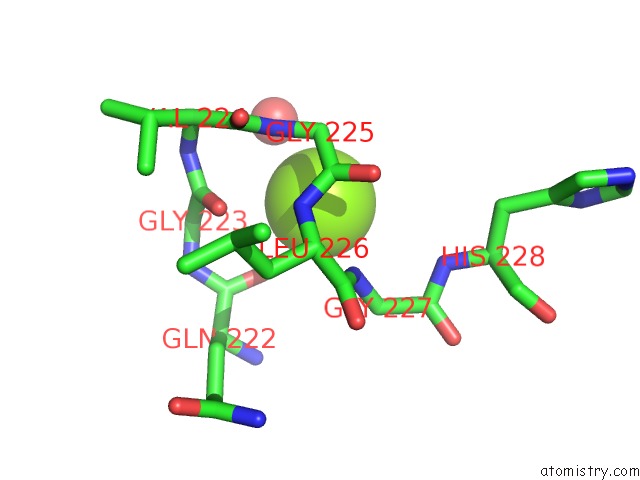
Mono view
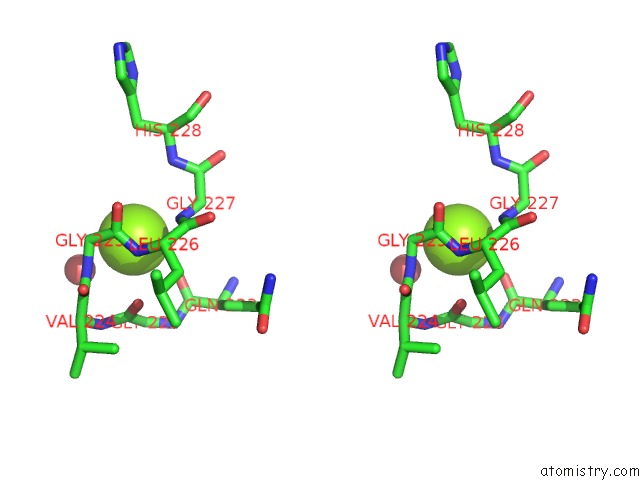
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 2 out of 7 in 1lnz
Go back to
Magnesium binding site 2 out
of 7 in the Structure of the Obg Gtp-Binding Protein
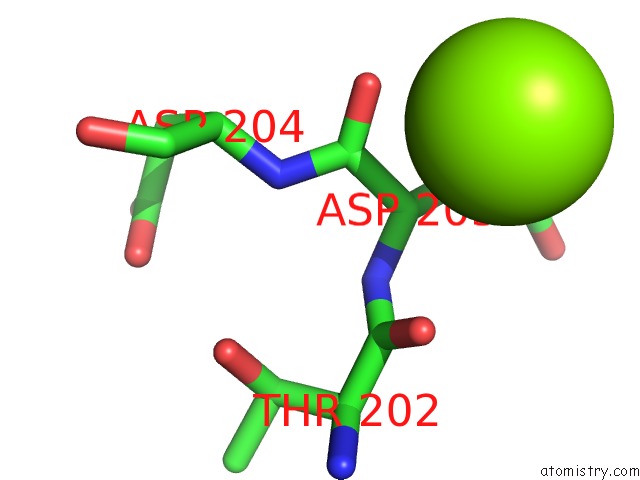
Mono view
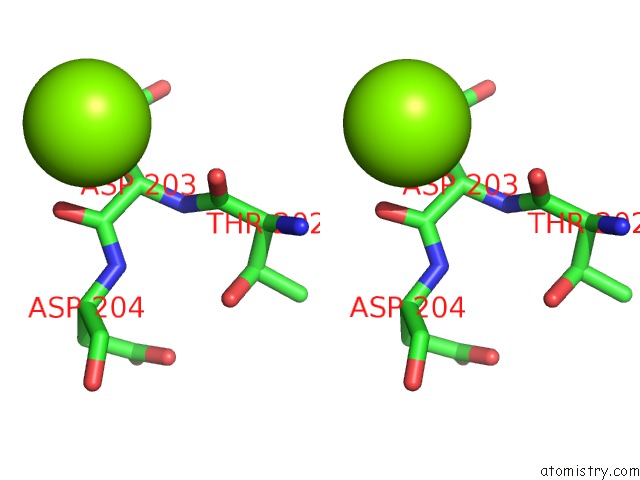
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 3 out of 7 in 1lnz
Go back to
Magnesium binding site 3 out
of 7 in the Structure of the Obg Gtp-Binding Protein
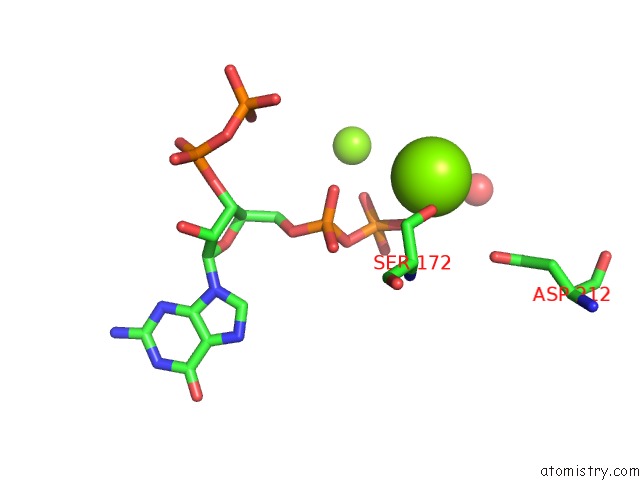
Mono view
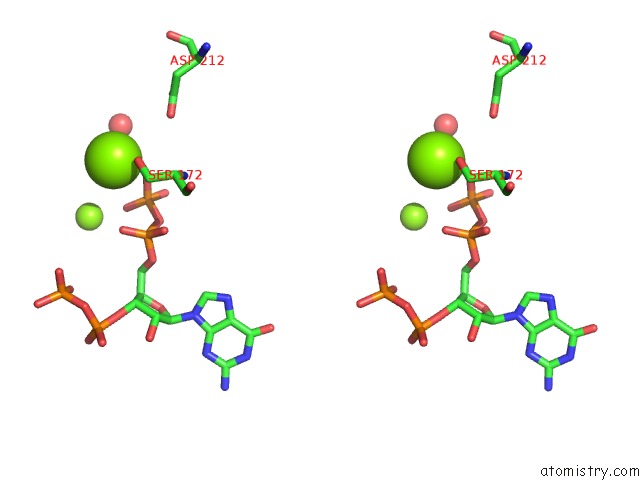
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 4 out of 7 in 1lnz
Go back to
Magnesium binding site 4 out
of 7 in the Structure of the Obg Gtp-Binding Protein
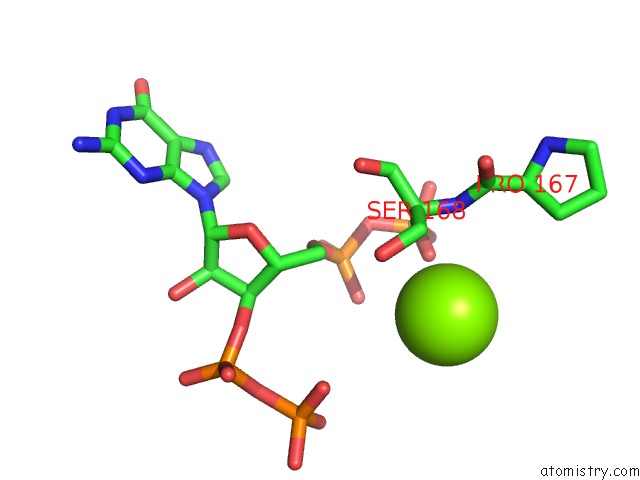
Mono view
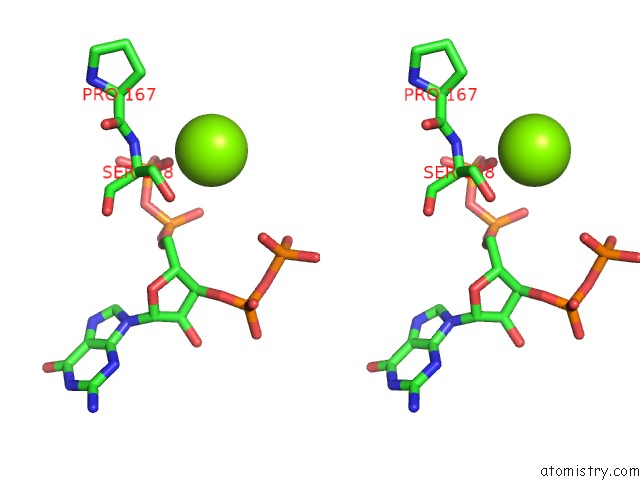
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 5 out of 7 in 1lnz
Go back to
Magnesium binding site 5 out
of 7 in the Structure of the Obg Gtp-Binding Protein
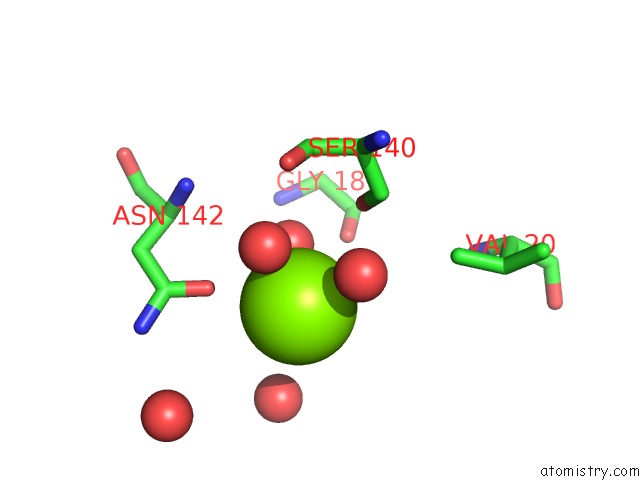
Mono view
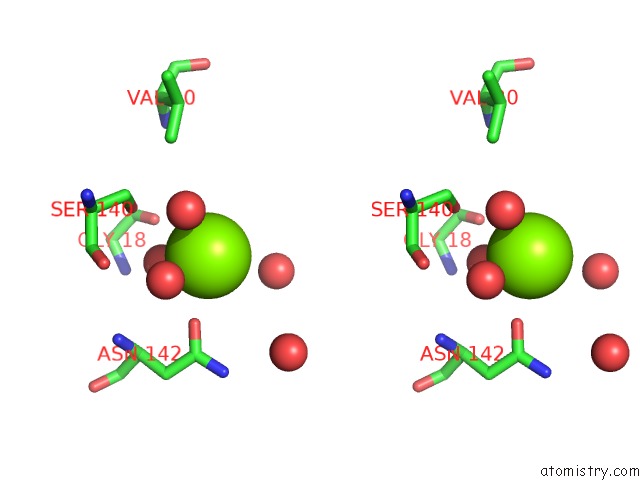
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 6 out of 7 in 1lnz
Go back to
Magnesium binding site 6 out
of 7 in the Structure of the Obg Gtp-Binding Protein
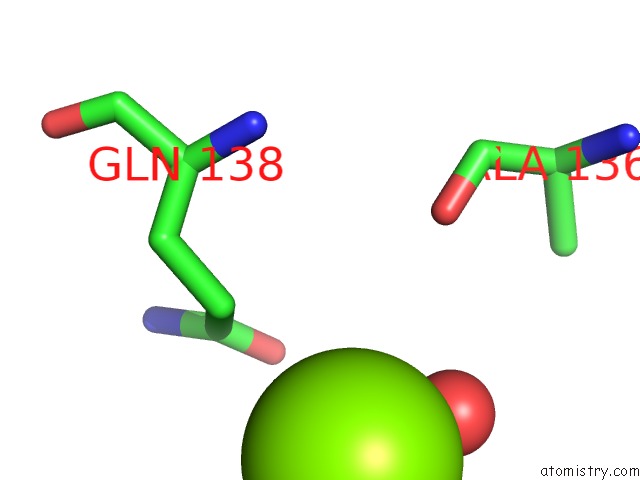
Mono view
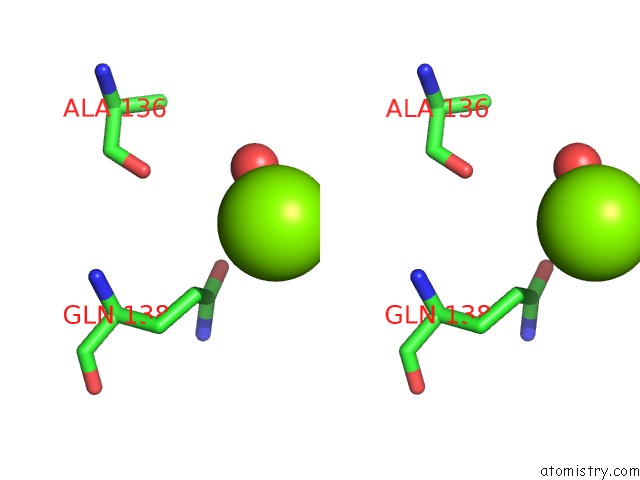
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Magnesium binding site 7 out of 7 in 1lnz
Go back to
Magnesium binding site 7 out
of 7 in the Structure of the Obg Gtp-Binding Protein
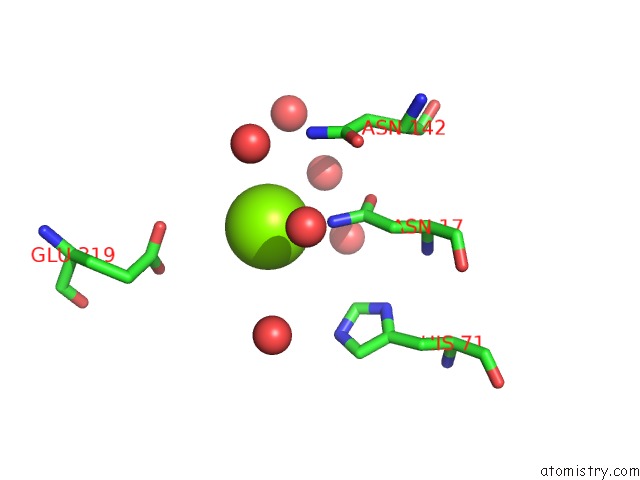
Mono view
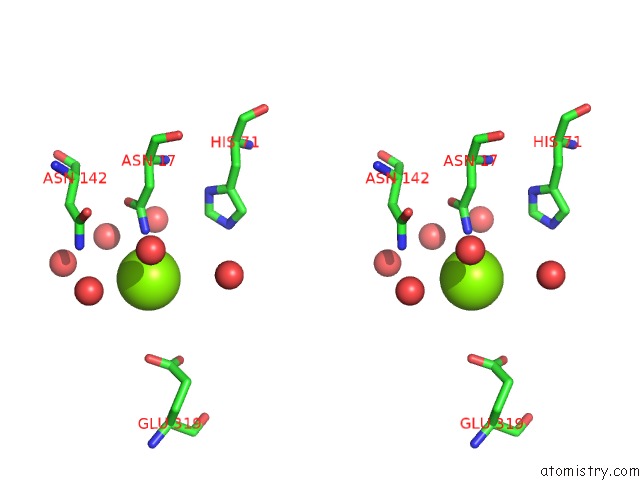
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Structure of the Obg Gtp-Binding Protein within 5.0Å range:
|
Reference:
J.Buglino,
V.Shen,
P.Hakimian,
C.D.Lima.
Structural and Biochemical Analysis of the Obg Gtp Binding Protein Structure V. 10 1581 2002.
ISSN: ISSN 0969-2126
PubMed: 12429099
DOI: 10.1016/S0969-2126(02)00882-1
Page generated: Sun Aug 10 00:44:00 2025
ISSN: ISSN 0969-2126
PubMed: 12429099
DOI: 10.1016/S0969-2126(02)00882-1
Last articles
Mg in 4FRZMg in 4FQF
Mg in 4FR3
Mg in 4FPV
Mg in 4FNJ
Mg in 4FPP
Mg in 4FP1
Mg in 4FO6
Mg in 4FO0
Mg in 4FME