Magnesium »
PDB 1x1t-1xfx »
1xbz »
Magnesium in PDB 1xbz: Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate
Protein crystallography data
The structure of Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate, PDB code: 1xbz
was solved by
E.L.Wise,
W.S.Yew,
J.Akana,
J.A.Gerlt,
I.Rayment,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 91.29 / 1.80 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 123.776, 41.435, 91.011, 90.00, 96.72, 90.00 |
| R / Rfree (%) | 16.8 / 21 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate
(pdb code 1xbz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate, PDB code: 1xbz:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate, PDB code: 1xbz:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 1xbz
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate
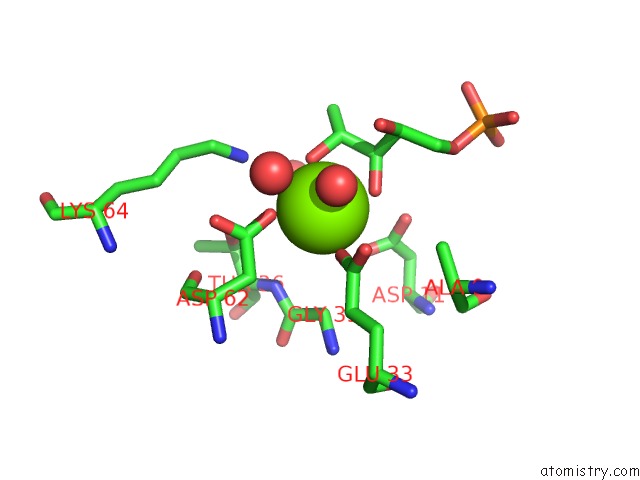
Mono view
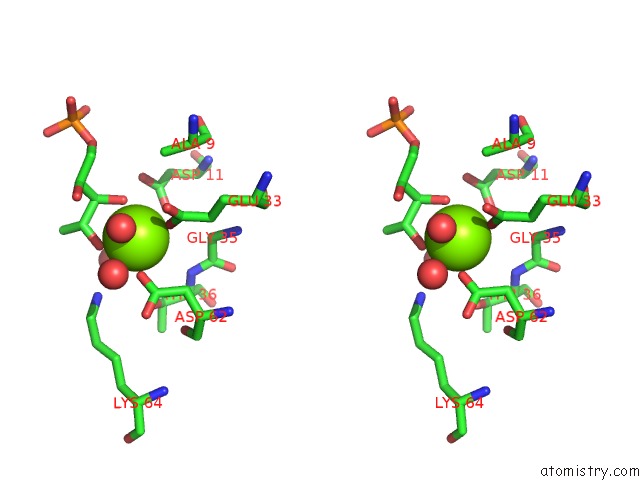
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 1xbz
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate
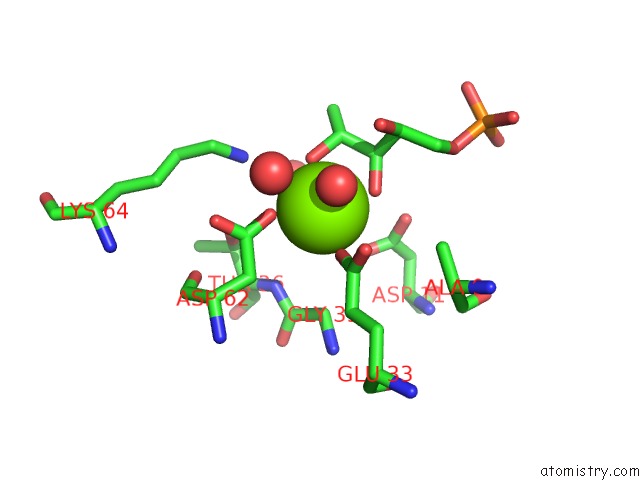
Mono view
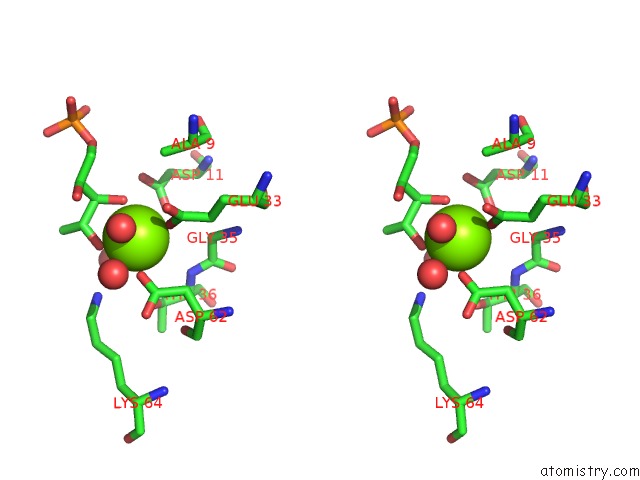
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase E112D/R139V/T169A Mutant with Bound L-Xylulose 5-Phosphate within 5.0Å range:
|
Reference:
E.L.Wise,
W.S.Yew,
J.Akana,
J.A.Gerlt,
I.Rayment.
Evolution of Enzymatic Activities in the Orotidine 5'-Monophosphate Decarboxylase Suprafamily: Structural Basis For Catalytic Promiscuity in Wild-Type and Designed Mutants of 3-Keto-L-Gulonate 6-Phosphate Decarboxylase Biochemistry V. 44 1816 2005.
ISSN: ISSN 0006-2960
PubMed: 15697207
DOI: 10.1021/BI0478143
Page generated: Sun Aug 10 06:57:57 2025
ISSN: ISSN 0006-2960
PubMed: 15697207
DOI: 10.1021/BI0478143
Last articles
Mg in 6EG8Mg in 6EHJ
Mg in 6EHH
Mg in 6EHE
Mg in 6EGF
Mg in 6EER
Mg in 6EFG
Mg in 6EFX
Mg in 6EFN
Mg in 6EED