Magnesium »
PDB 1xyl-1ydf »
1y9d »
Magnesium in PDB 1y9d: Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
Enzymatic activity of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
All present enzymatic activity of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum:
1.2.3.3;
1.2.3.3;
Protein crystallography data
The structure of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum, PDB code: 1y9d
was solved by
G.Wille,
M.Ritter,
M.S.Weiss,
S.Konig,
W.Mantele,
G.Hubner,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 27.30 / 2.20 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 94.660, 155.780, 100.750, 90.00, 92.92, 90.00 |
| R / Rfree (%) | 17.8 / 23.8 |
Other elements in 1y9d:
The structure of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum also contains other interesting chemical elements:
| Sodium | (Na) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
(pdb code 1y9d). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum, PDB code: 1y9d:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum, PDB code: 1y9d:
Jump to Magnesium binding site number: 1; 2; 3; 4;
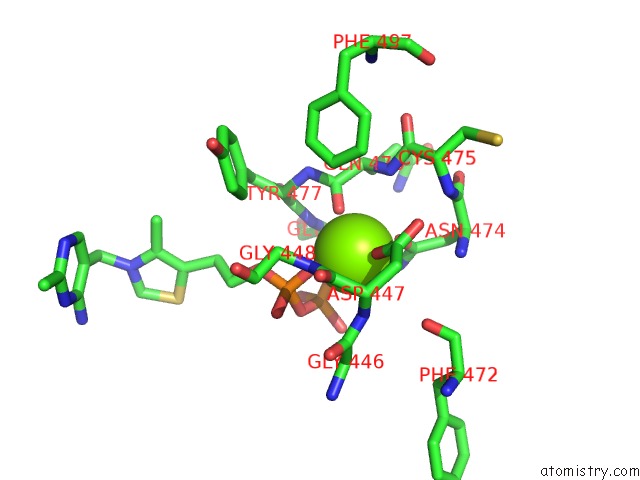
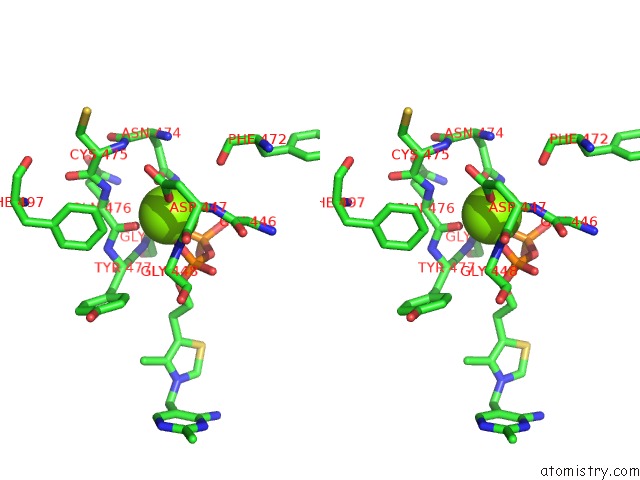
Magnesium binding site 1 out of 4 in 1y9d
Go back to
Magnesium binding site 1 out
of 4 in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum within 5.0Å range:
|
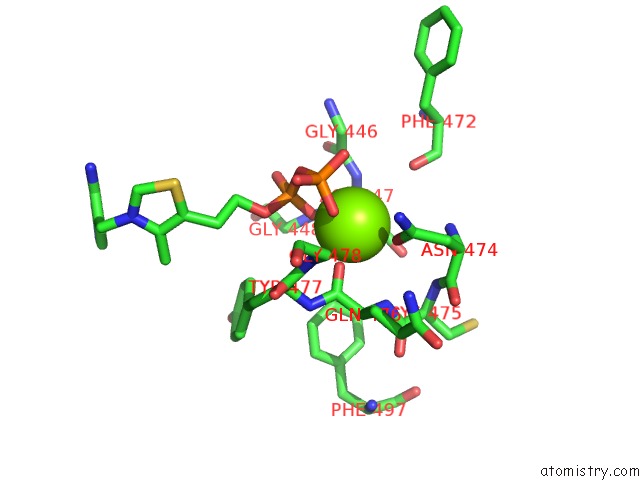
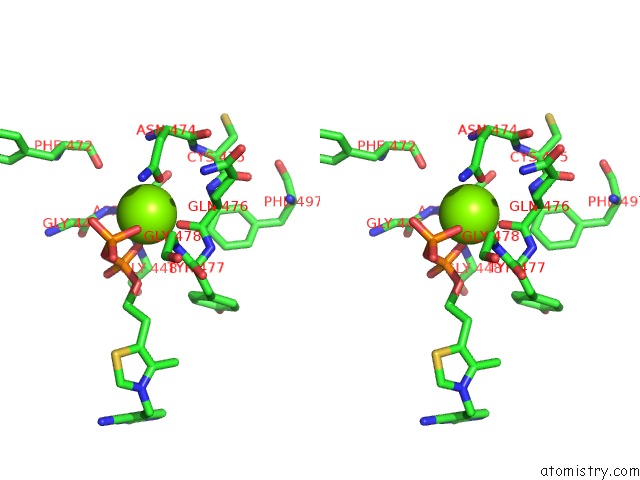
Magnesium binding site 2 out of 4 in 1y9d
Go back to
Magnesium binding site 2 out
of 4 in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 1y9d
Go back to
Magnesium binding site 3 out
of 4 in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
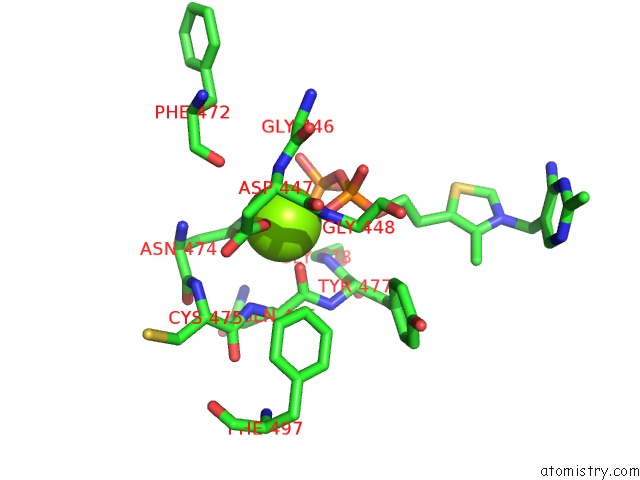
Mono view
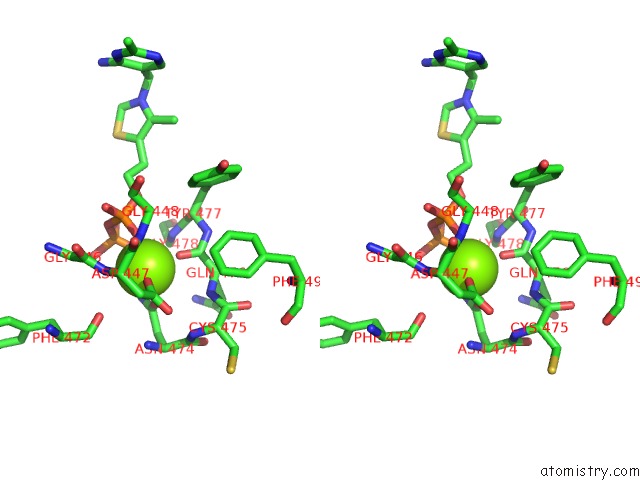
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 1y9d
Go back to
Magnesium binding site 4 out
of 4 in the Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum
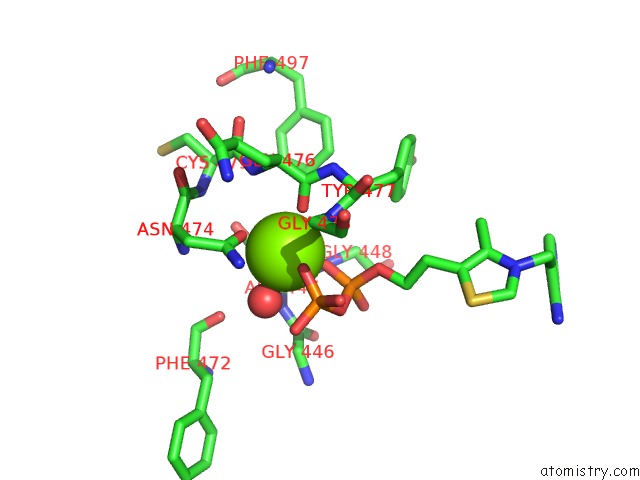
Mono view
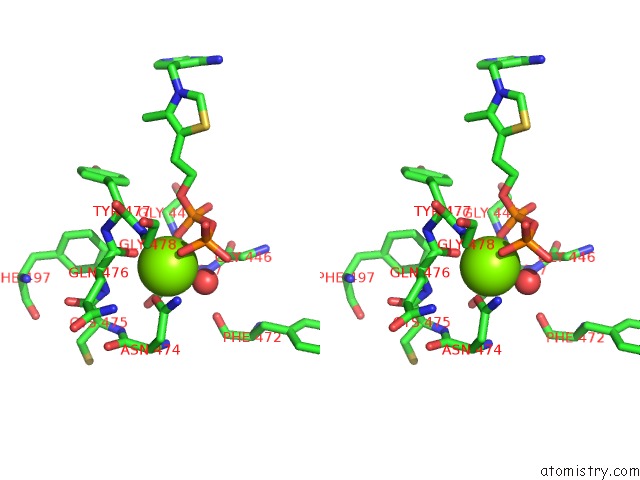
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Pyruvate Oxidase Variant V265A From Lactobacillus Plantarum within 5.0Å range:
|
Reference:
G.Wille,
M.Ritter,
M.S.Weiss,
S.Konig,
W.Mantele,
G.Hubner.
The Role of Val-265 For Flavin Adenine Dinulceotide (Fad) Binding in Pyruvate Oxidase: Ftir, Kinetic and Crystallographic Studies on the Enzyme Variant V265A Biochemistry V. 44 5086 2005.
ISSN: ISSN 0006-2960
PubMed: 15794646
DOI: 10.1021/BI047337O
Page generated: Sun Aug 10 07:44:16 2025
ISSN: ISSN 0006-2960
PubMed: 15794646
DOI: 10.1021/BI047337O
Last articles
Mg in 3CC2Mg in 3CCE
Mg in 3CC7
Mg in 3CC4
Mg in 3CB3
Mg in 3CC6
Mg in 3C9U
Mg in 3CBT
Mg in 3CBQ
Mg in 3CBG