Magnesium »
PDB 3i5y-3ig8 »
3ig8 »
Magnesium in PDB 3ig8: Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
Enzymatic activity of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
All present enzymatic activity of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp:
6.3.2.2;
6.3.2.2;
Protein crystallography data
The structure of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp, PDB code: 3ig8
was solved by
E.Biterova,
J.J.Barycki,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 33.90 / 2.69 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 117.849, 117.849, 164.444, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.7 / 24.2 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
(pdb code 3ig8). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp, PDB code: 3ig8:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp, PDB code: 3ig8:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 3ig8
Go back to
Magnesium binding site 1 out
of 3 in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
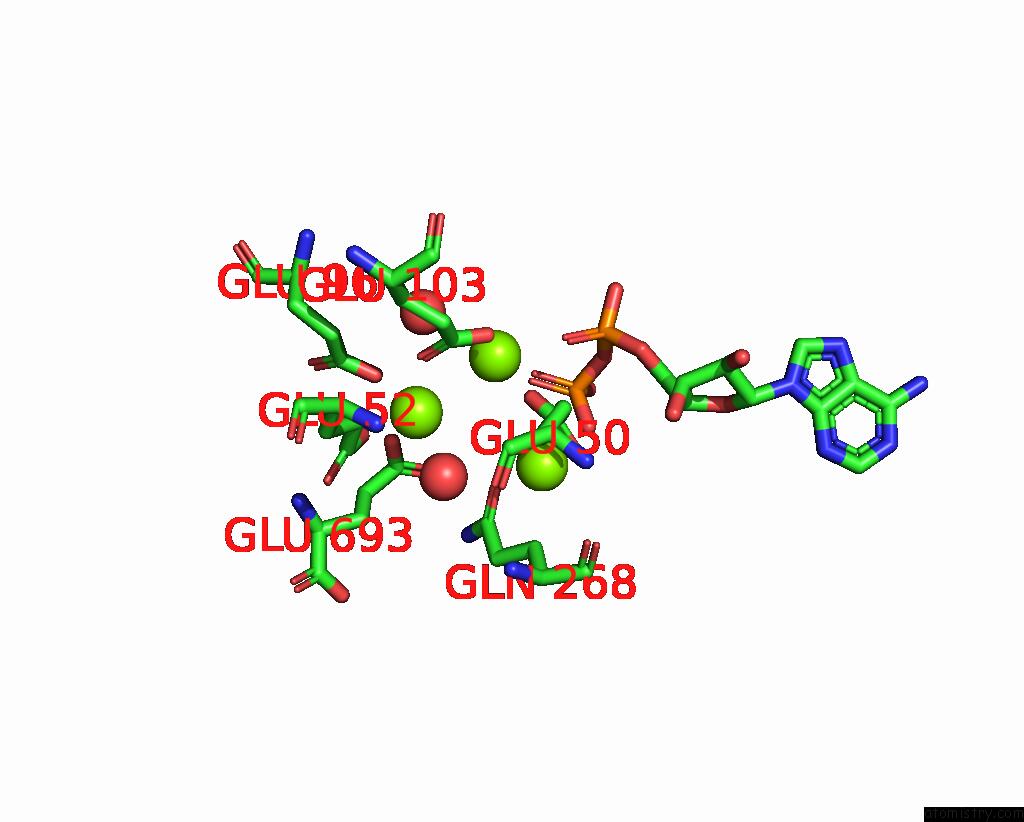
Mono view
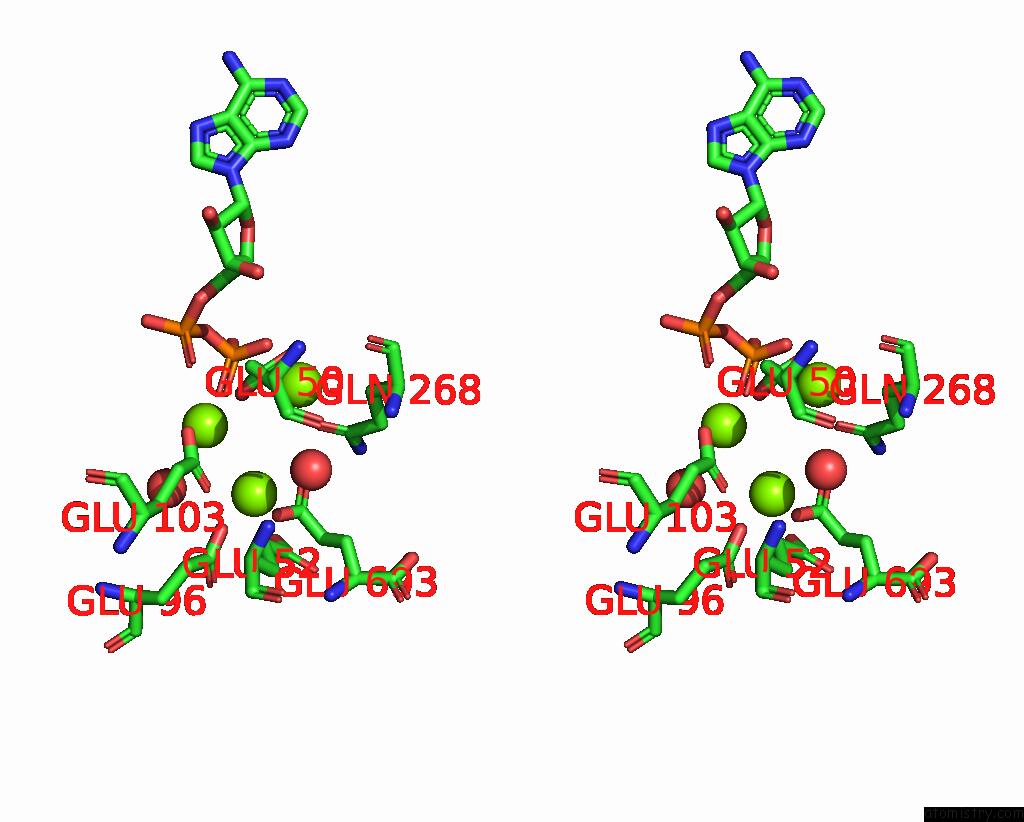
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 3ig8
Go back to
Magnesium binding site 2 out
of 3 in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
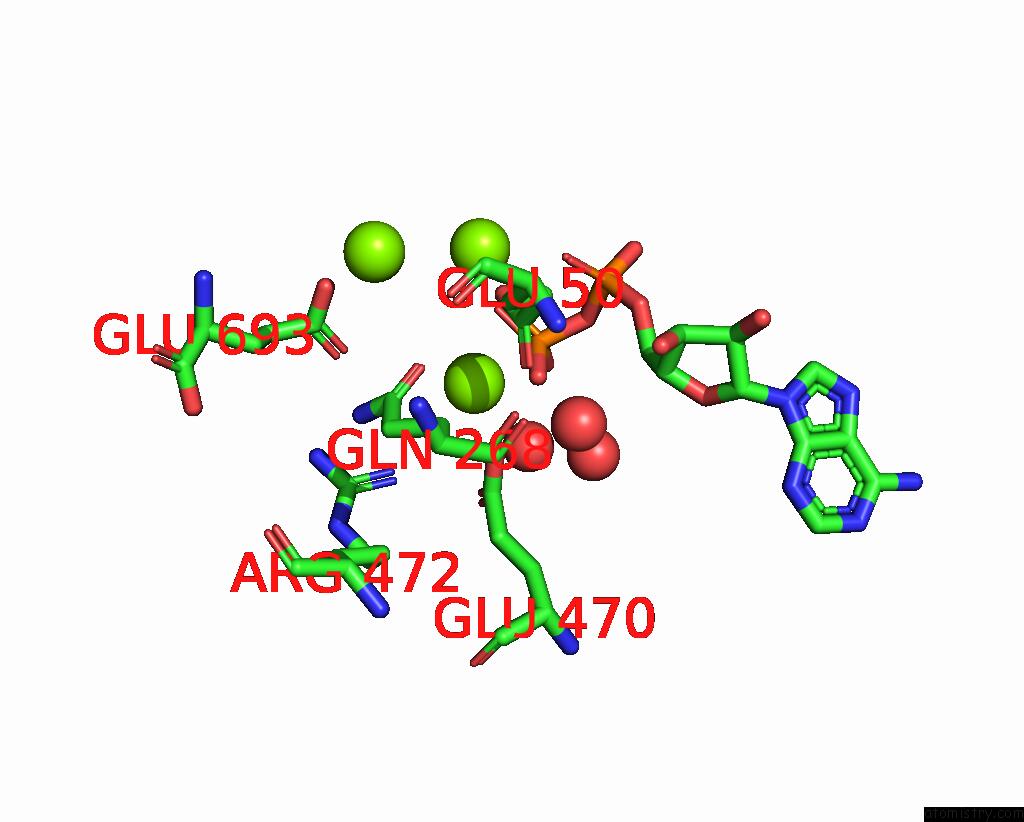
Mono view
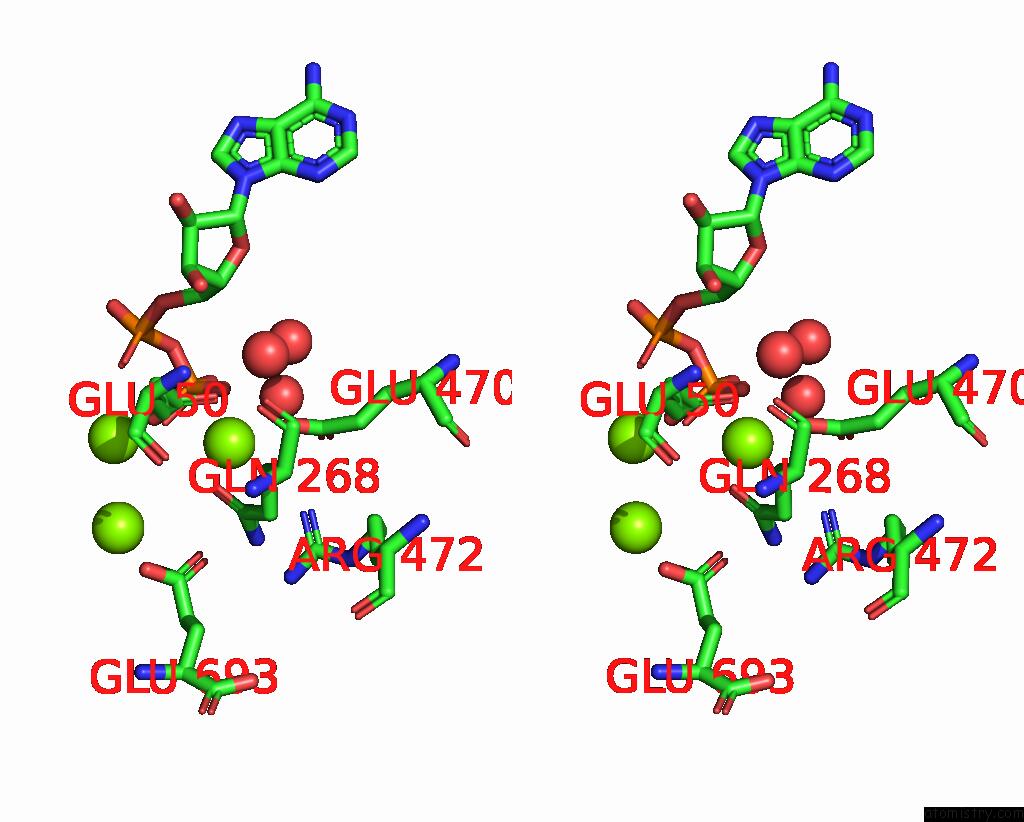
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 3ig8
Go back to
Magnesium binding site 3 out
of 3 in the Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp
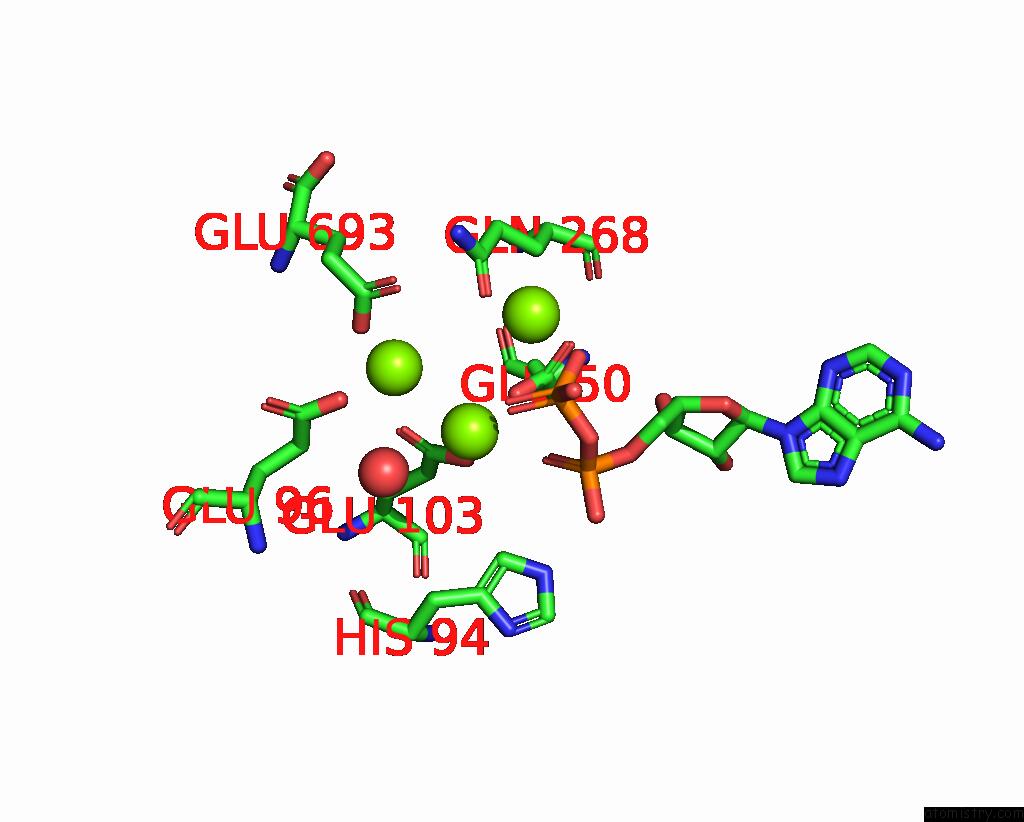
Mono view
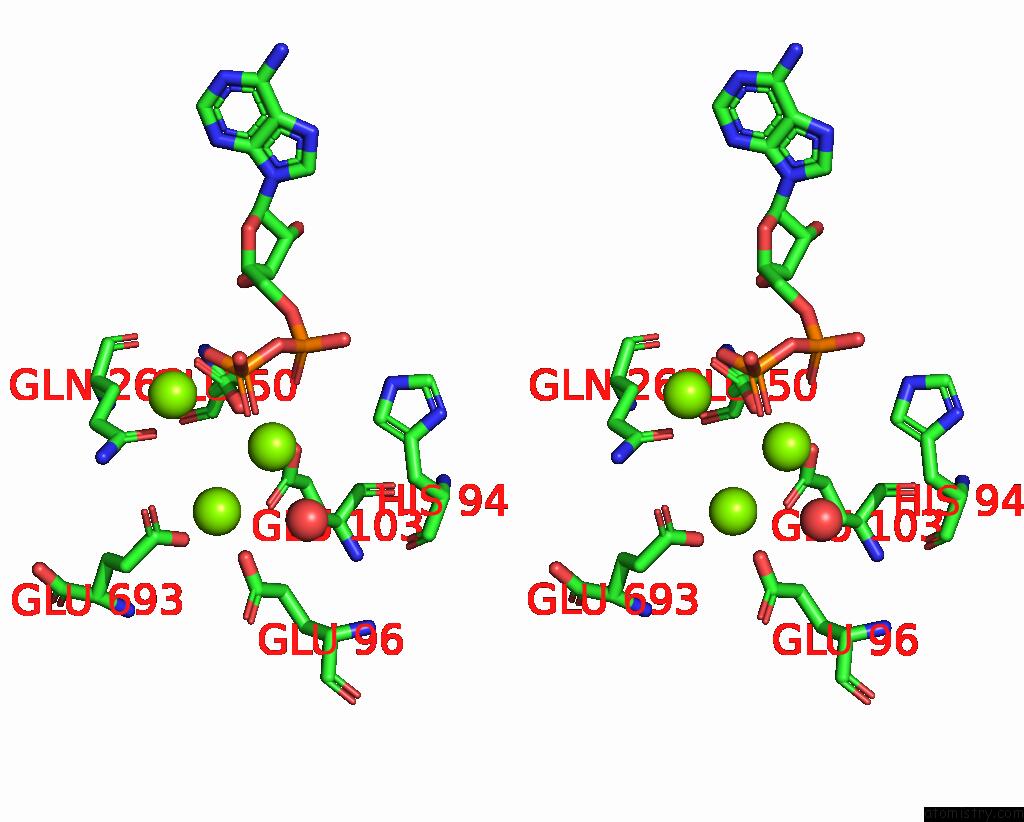
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Saccharomyces Cerevisiae Glutamate Cysteine Ligase in Complex with MG2+, L-Glutamate and Adp within 5.0Å range:
|
Reference:
E.I.Biterova,
J.J.Barycki.
Mechanistic Details of Glutathione Biosynthesis Revealed By Crystal Structures of Saccharomyces Cerevisiae Glutamate Cysteine Ligase. J.Biol.Chem. V. 284 32700 2009.
ISSN: ISSN 0021-9258
PubMed: 19726687
DOI: 10.1074/JBC.M109.025114
Page generated: Sun Aug 10 22:34:57 2025
ISSN: ISSN 0021-9258
PubMed: 19726687
DOI: 10.1074/JBC.M109.025114
Last articles
Mg in 6YBWMg in 6YKW
Mg in 6YKV
Mg in 6YKU
Mg in 6YKT
Mg in 6YKS
Mg in 6YKQ
Mg in 6YKO
Mg in 6YKN
Mg in 6YKL