Magnesium »
PDB 3s99-3si8 »
3sep »
Magnesium in PDB 3sep: E. Coli (Lacz) Beta-Galactosidase (S796A)
Enzymatic activity of E. Coli (Lacz) Beta-Galactosidase (S796A)
All present enzymatic activity of E. Coli (Lacz) Beta-Galactosidase (S796A):
3.2.1.23;
3.2.1.23;
Protein crystallography data
The structure of E. Coli (Lacz) Beta-Galactosidase (S796A), PDB code: 3sep
was solved by
L.J.Jancewicz,
R.W.Wheatley,
G.Sutendra,
M.Lee,
M.Fraser,
R.E.Huber,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 80.00 / 2.05 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 152.342, 163.165, 203.401, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.2 / 22.1 |
Other elements in 3sep:
The structure of E. Coli (Lacz) Beta-Galactosidase (S796A) also contains other interesting chemical elements:
| Sodium | (Na) | 14 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the E. Coli (Lacz) Beta-Galactosidase (S796A)
(pdb code 3sep). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 9 binding sites of Magnesium where determined in the E. Coli (Lacz) Beta-Galactosidase (S796A), PDB code: 3sep:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9;
In total 9 binding sites of Magnesium where determined in the E. Coli (Lacz) Beta-Galactosidase (S796A), PDB code: 3sep:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9;
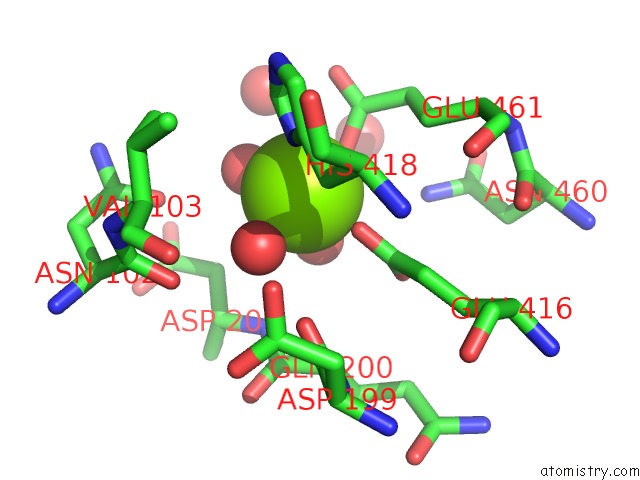
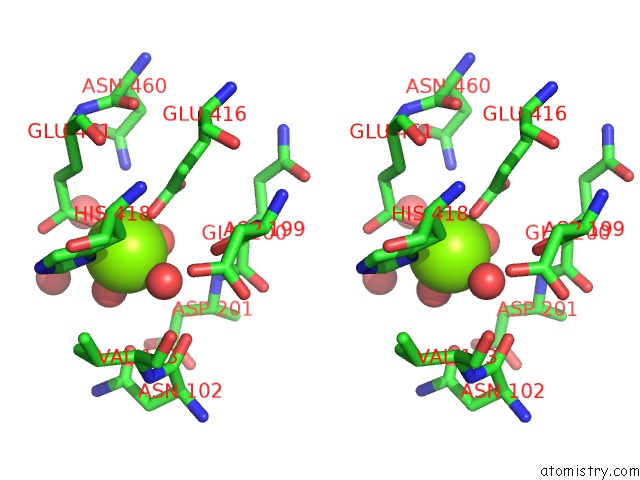
Magnesium binding site 1 out of 9 in 3sep
Go back to
Magnesium binding site 1 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
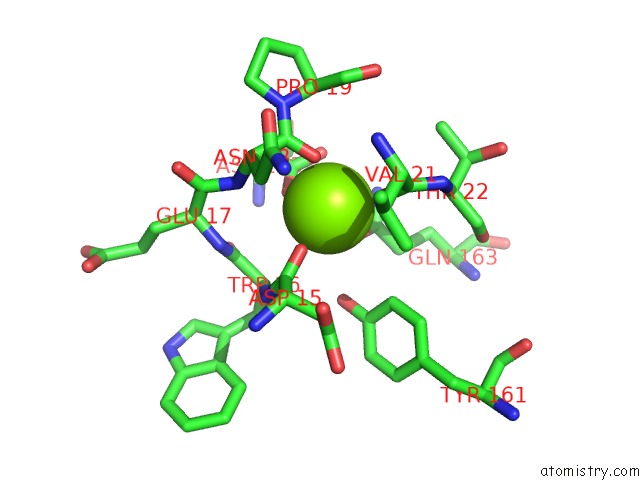
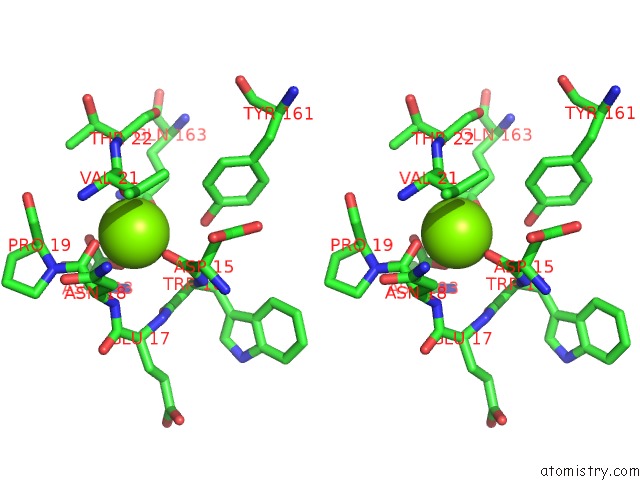
Magnesium binding site 2 out of 9 in 3sep
Go back to
Magnesium binding site 2 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
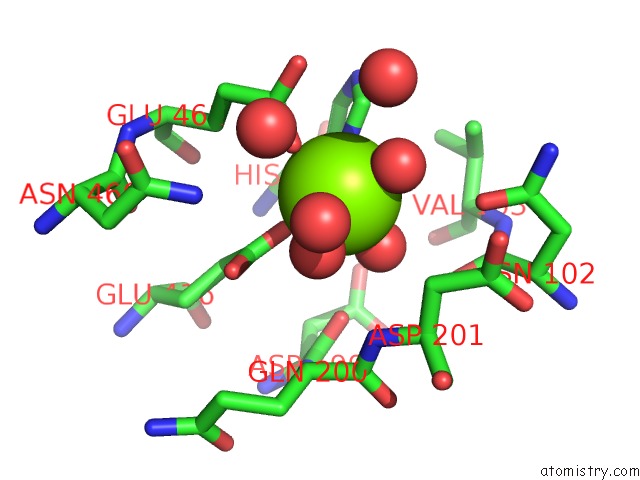
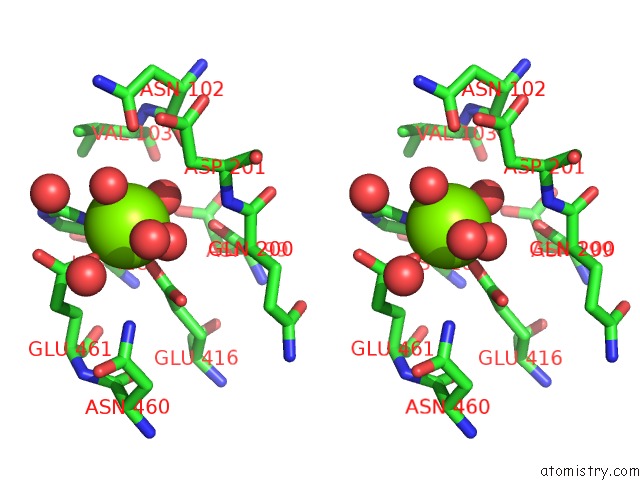
Magnesium binding site 3 out of 9 in 3sep
Go back to
Magnesium binding site 3 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
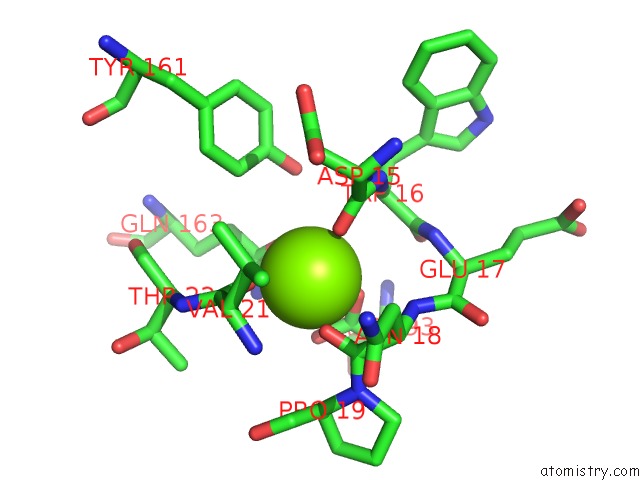
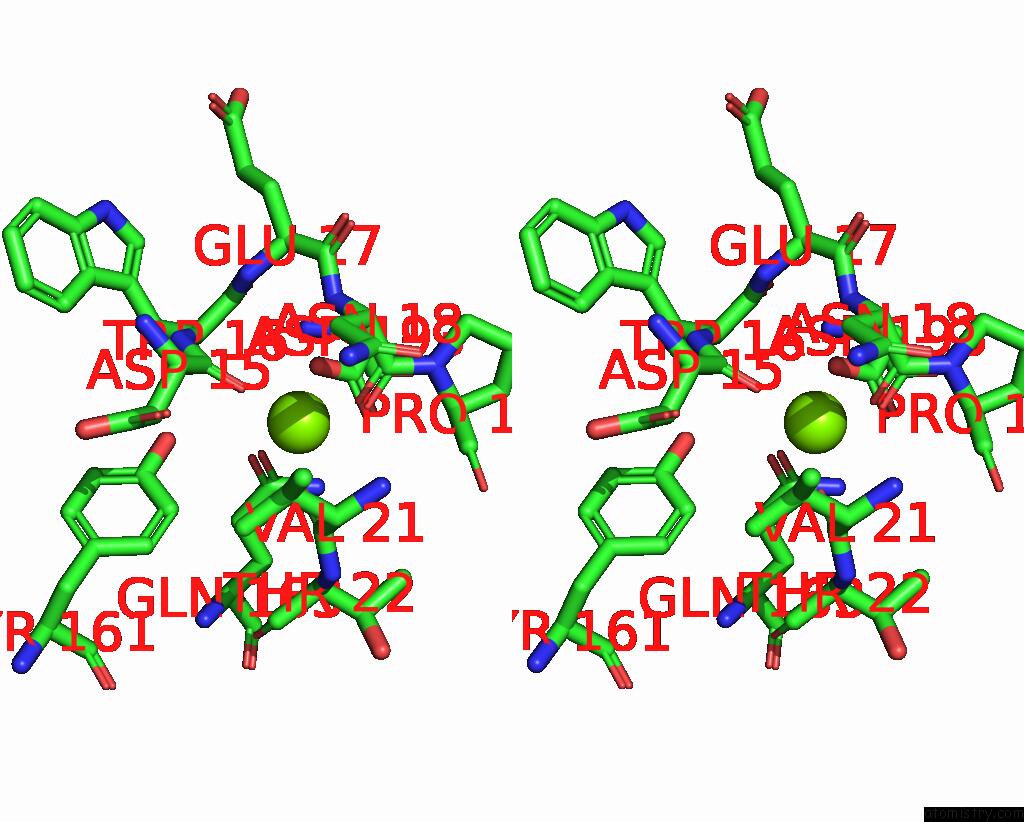
Magnesium binding site 4 out of 9 in 3sep
Go back to
Magnesium binding site 4 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
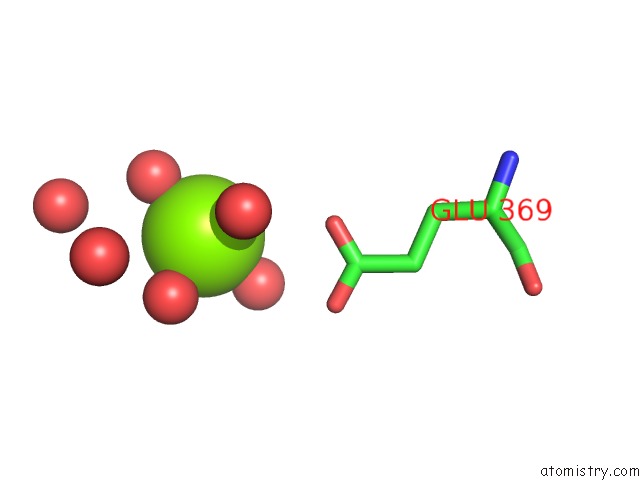
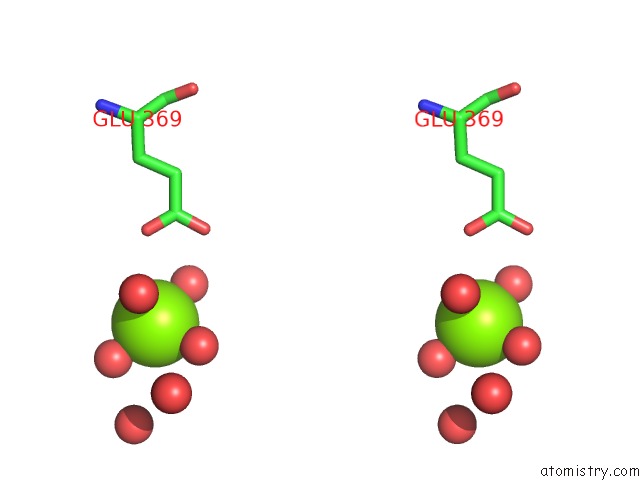
Magnesium binding site 5 out of 9 in 3sep
Go back to
Magnesium binding site 5 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
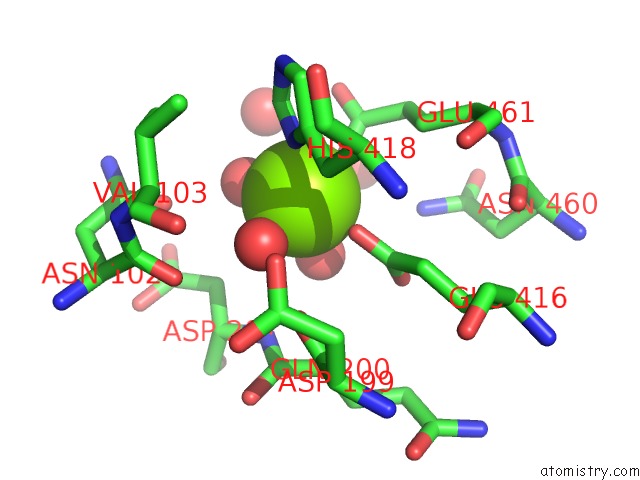
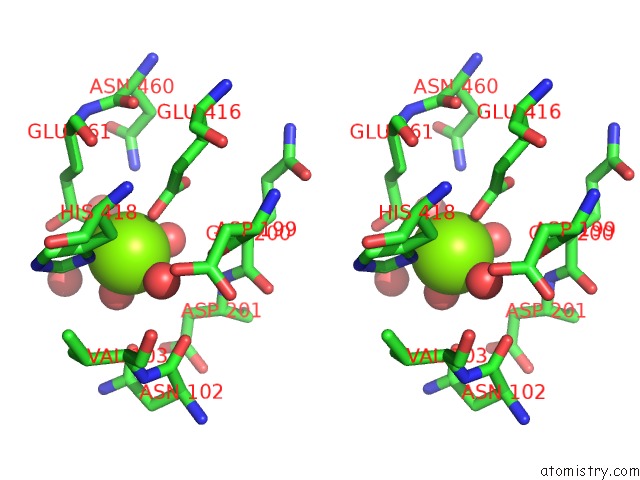
Magnesium binding site 6 out of 9 in 3sep
Go back to
Magnesium binding site 6 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
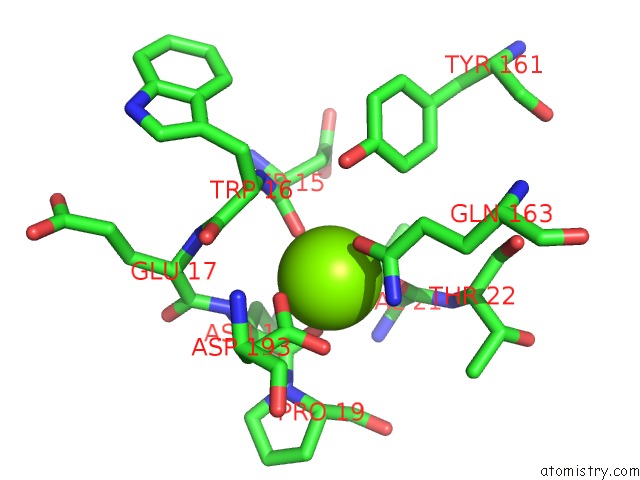
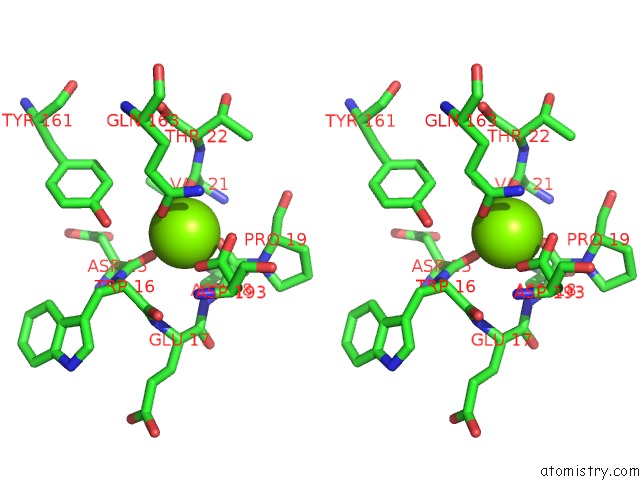
Magnesium binding site 7 out of 9 in 3sep
Go back to
Magnesium binding site 7 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
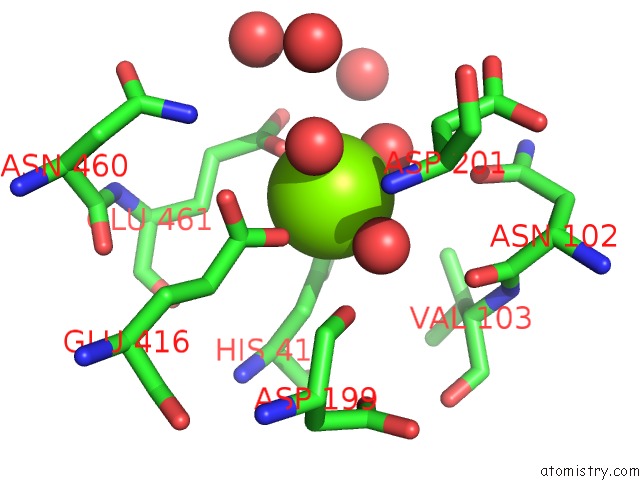
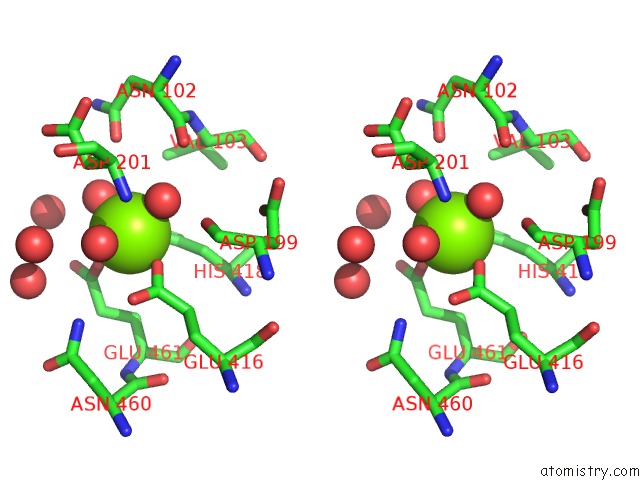
Magnesium binding site 8 out of 9 in 3sep
Go back to
Magnesium binding site 8 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
Magnesium binding site 9 out of 9 in 3sep
Go back to
Magnesium binding site 9 out
of 9 in the E. Coli (Lacz) Beta-Galactosidase (S796A)
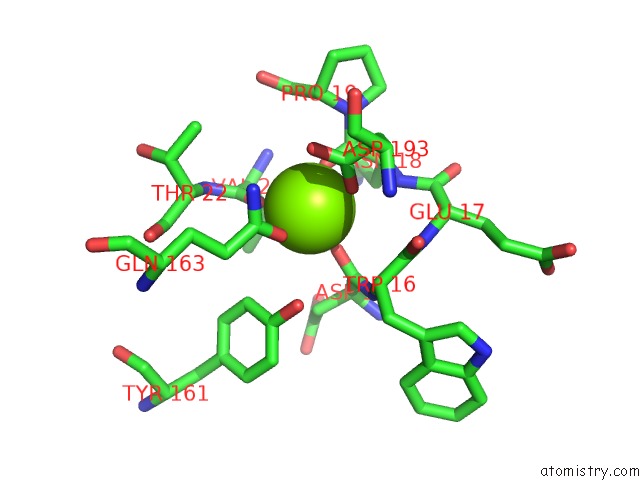
Mono view
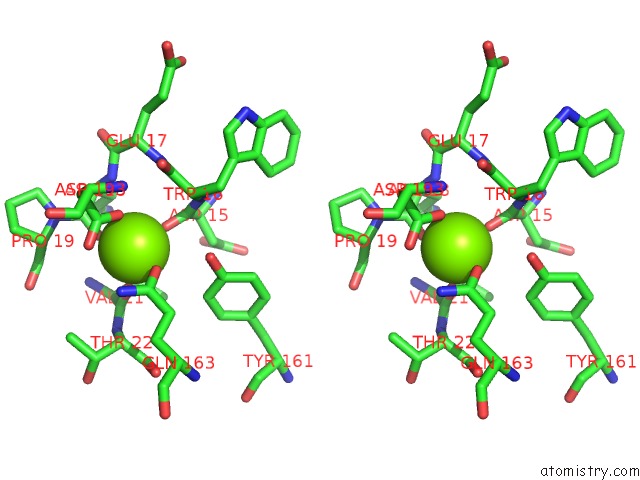
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 9 of E. Coli (Lacz) Beta-Galactosidase (S796A) within 5.0Å range:
|
Reference:
L.J.Jancewicz,
R.W.Wheatley,
G.Sutendra,
M.Lee,
M.E.Fraser,
R.E.Huber.
Ser-796 of Beta-Galactosidase (E. Coli) Plays A Key Role in Maintaining An Optimum Balance Between the Opened and Closed Conformations of the Catalytically Important Active Site Loop Arch.Biochem.Biophys. V. 517 111 2012.
ISSN: ISSN 0003-9861
PubMed: 22155115
DOI: 10.1016/J.ABB.2011.11.017
Page generated: Mon Aug 11 03:03:26 2025
ISSN: ISSN 0003-9861
PubMed: 22155115
DOI: 10.1016/J.ABB.2011.11.017
Last articles
Mg in 4FR8Mg in 4FS1
Mg in 4FRZ
Mg in 4FQF
Mg in 4FR3
Mg in 4FPV
Mg in 4FNJ
Mg in 4FPP
Mg in 4FP1
Mg in 4FO6