Magnesium »
PDB 3wdl-3wqc »
3wnz »
Magnesium in PDB 3wnz: Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi
Enzymatic activity of Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi
All present enzymatic activity of Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi:
6.3.2.28;
6.3.2.28;
Protein crystallography data
The structure of Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi, PDB code: 3wnz
was solved by
T.Tsuda,
S.Kojima,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 17.42 / 1.90 |
| Space group | P 65 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 90.581, 90.581, 252.654, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.7 / 24.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi
(pdb code 3wnz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi, PDB code: 3wnz:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi, PDB code: 3wnz:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 3wnz
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi
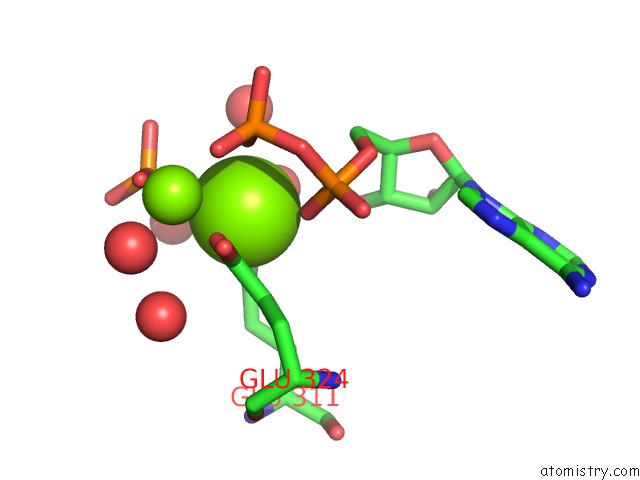
Mono view
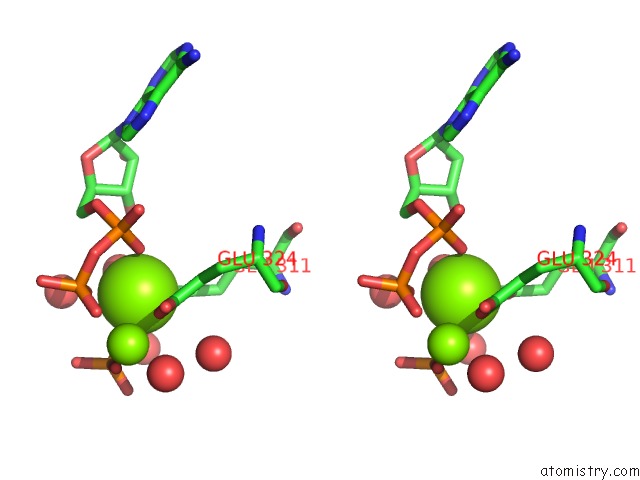
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 3wnz
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi
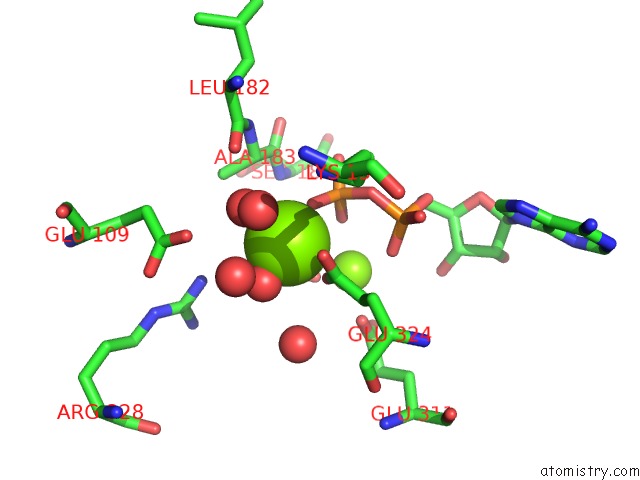
Mono view
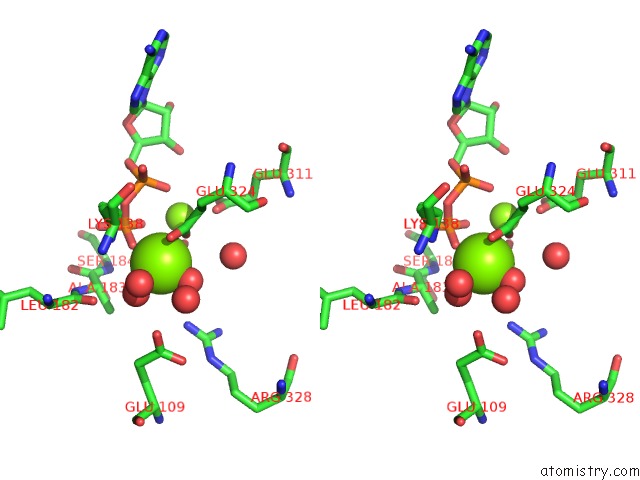
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Bacillus Subtilis Ywfe, An L-Amino Acid Ligase, with Bound Adp-Mg-Pi within 5.0Å range:
|
Reference:
T.Tsuda,
M.Asami,
Y.Koguchi,
S.Kojima.
Single Mutation Alters the Substrate Specificity of L-Amino Acid Ligase Biochemistry V. 53 2650 2014.
ISSN: ISSN 0006-2960
PubMed: 24702628
DOI: 10.1021/BI500292B
Page generated: Mon Aug 11 04:55:07 2025
ISSN: ISSN 0006-2960
PubMed: 24702628
DOI: 10.1021/BI500292B
Last articles
Mg in 5BRPMg in 5BRN
Mg in 5BQD
Mg in 5BRJ
Mg in 5BQ2
Mg in 5BOU
Mg in 5BQ5
Mg in 5BPJ
Mg in 5BPM
Mg in 5BPL