Magnesium »
PDB 3wqd-3x1l »
3wy4 »
Magnesium in PDB 3wy4: Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose
Enzymatic activity of Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose
All present enzymatic activity of Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose:
3.2.1.20;
3.2.1.20;
Protein crystallography data
The structure of Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose, PDB code: 3wy4
was solved by
X.Shen,
Z.Gai,
K.Kato,
M.Yao,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.46 / 2.50 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 111.464, 181.062, 51.933, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.2 / 24.1 |
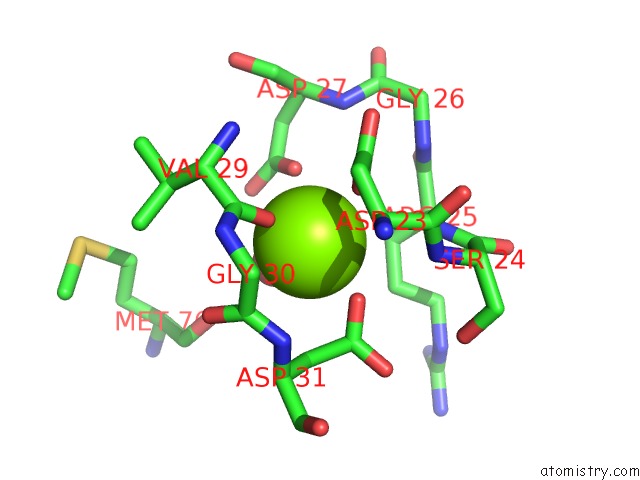
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose
(pdb code 3wy4). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose, PDB code: 3wy4:
In total only one binding site of Magnesium was determined in the Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose, PDB code: 3wy4:
Magnesium binding site 1 out of 1 in 3wy4
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose
Mono view
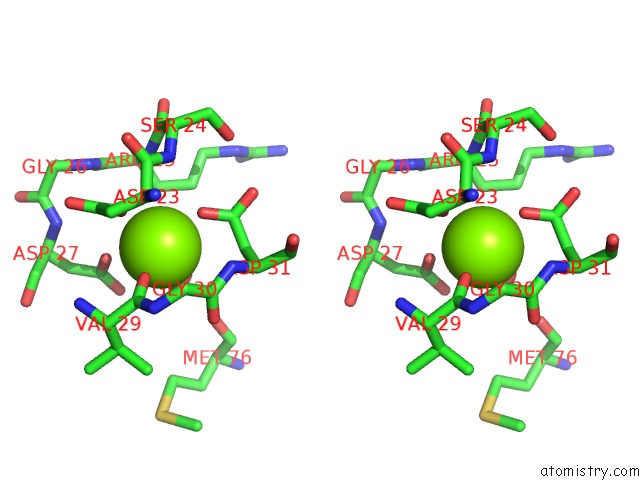
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Alpha-Glucosidase Mutant E271Q in Complex with Maltose within 5.0Å range:
|
Reference:
X.Shen,
W.Saburi,
Z.Gai,
K.Kato,
T.Ojima-Kato,
J.Yu,
K.Komoda,
Y.Kido,
H.Matsui,
H.Mori,
M.Yao.
Structural Analysis of the Alpha-Glucosidase Hag Provides New Insights Into Substrate Specificity and Catalytic Mechanism Acta Crystallogr. D Biol. V. 71 1382 2015CRYSTALLOGR..
ISSN: ESSN 1399-0047
PubMed: 26057678
DOI: 10.1107/S139900471500721X
Page generated: Mon Aug 11 05:04:16 2025
ISSN: ESSN 1399-0047
PubMed: 26057678
DOI: 10.1107/S139900471500721X
Last articles
Mg in 6KOUMg in 6KPE
Mg in 6KOQ
Mg in 6KNZ
Mg in 6KOP
Mg in 6KON
Mg in 6KOO
Mg in 6KO6
Mg in 6KO1
Mg in 6KMA