Magnesium »
PDB 4erm-4f6x »
4euk »
Magnesium in PDB 4euk: Crystal Structure
Enzymatic activity of Crystal Structure
All present enzymatic activity of Crystal Structure:
2.7.13.3;
2.7.13.3;
Protein crystallography data
The structure of Crystal Structure, PDB code: 4euk
was solved by
T.Stehle,
J.Bauer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.76 / 1.95 |
| Space group | P 21 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 106.800, 106.800, 106.800, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.2 / 19.7 |
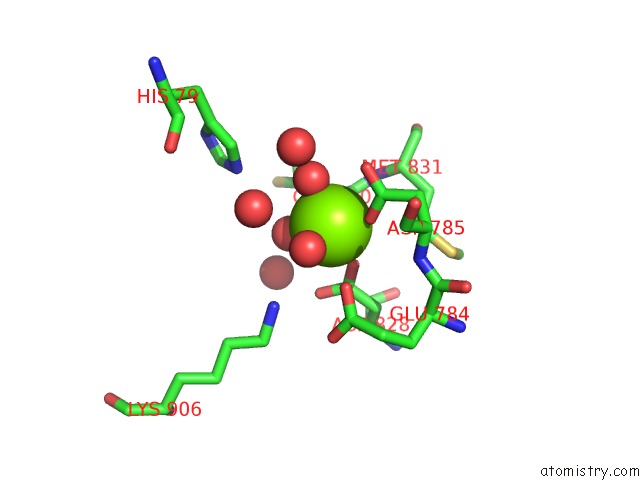
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure
(pdb code 4euk). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Crystal Structure, PDB code: 4euk:
In total only one binding site of Magnesium was determined in the Crystal Structure, PDB code: 4euk:
Magnesium binding site 1 out of 1 in 4euk
Go back to
Magnesium binding site 1 out
of 1 in the Crystal Structure
Mono view
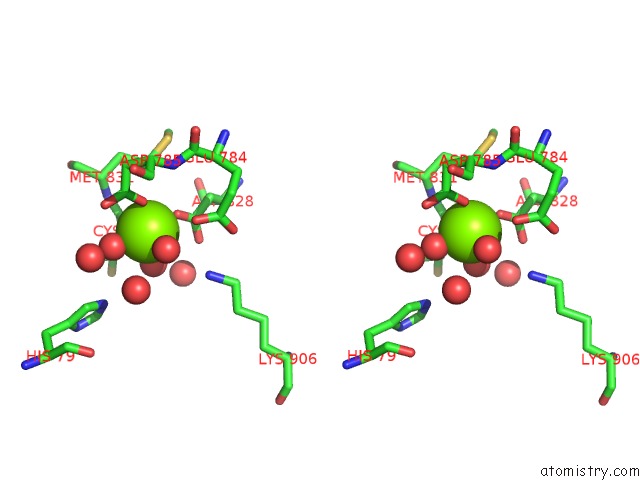
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure within 5.0Å range:
|
Reference:
J.Bauer,
K.Reiss,
M.Veerabagu,
M.Heunemann,
K.Harter,
T.Stehle.
Structure-Function Analysis of Arabidopsis Thaliana Histidine Kinase AHK5 Bound to Its Cognate Phosphotransfer Protein AHP1. Mol Plant V. 6 959 2013.
ISSN: ISSN 1674-2052
PubMed: 23132142
DOI: 10.1093/MP/SSS126
Page generated: Mon Aug 11 12:30:49 2025
ISSN: ISSN 1674-2052
PubMed: 23132142
DOI: 10.1093/MP/SSS126
Last articles
Mg in 4JHDMg in 4JH6
Mg in 4JH8
Mg in 4JH7
Mg in 4JH3
Mg in 4JH5
Mg in 4JF2
Mg in 4JH2
Mg in 4JH1
Mg in 4JEJ