Magnesium »
PDB 4ha3-4hlq »
4hgq »
Magnesium in PDB 4hgq: Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
Protein crystallography data
The structure of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron, PDB code: 4hgq
was solved by
K.D.Daughtry,
K.N.Allen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 37.10 / 2.28 |
| Space group | P 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.941, 94.116, 161.497, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.5 / 24.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
(pdb code 4hgq). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 8 binding sites of Magnesium where determined in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron, PDB code: 4hgq:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Magnesium where determined in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron, PDB code: 4hgq:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Magnesium binding site 1 out of 8 in 4hgq
Go back to
Magnesium binding site 1 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
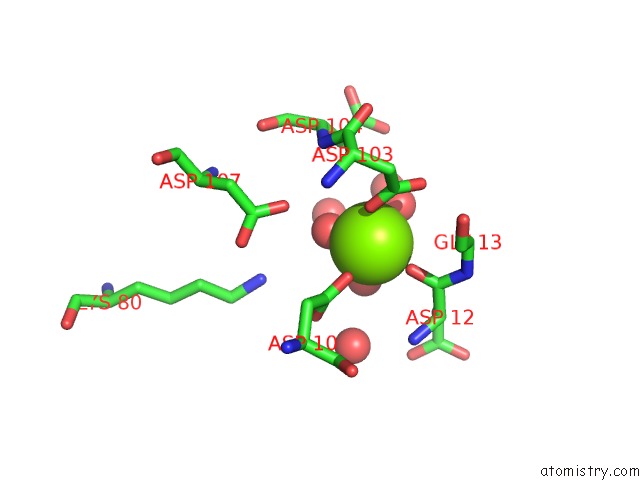
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
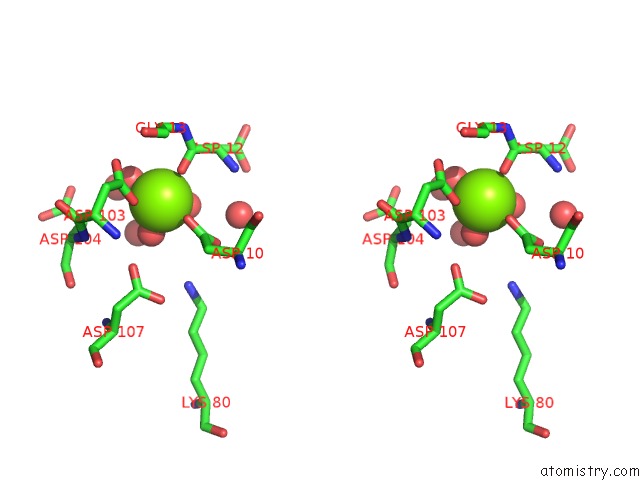
Magnesium binding site 2 out of 8 in 4hgq
Go back to
Magnesium binding site 2 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
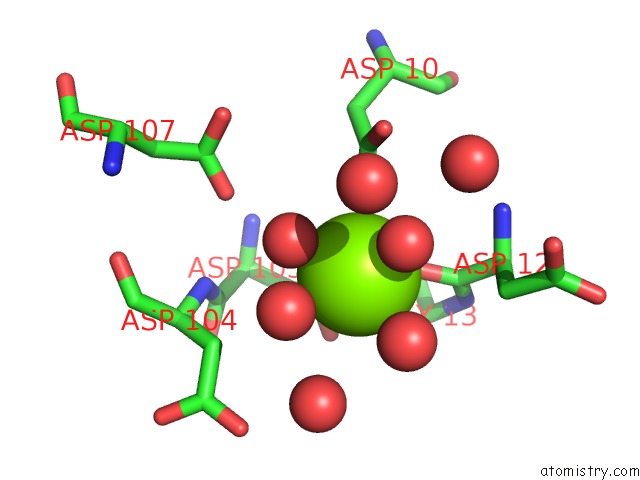
Magnesium binding site 3 out of 8 in 4hgq
Go back to
Magnesium binding site 3 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
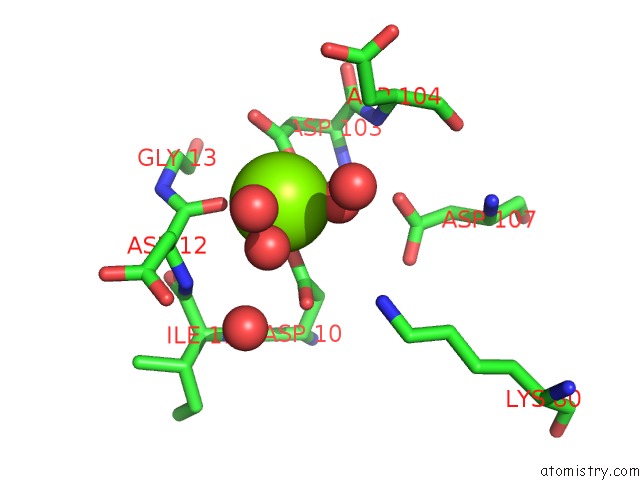
Mono view
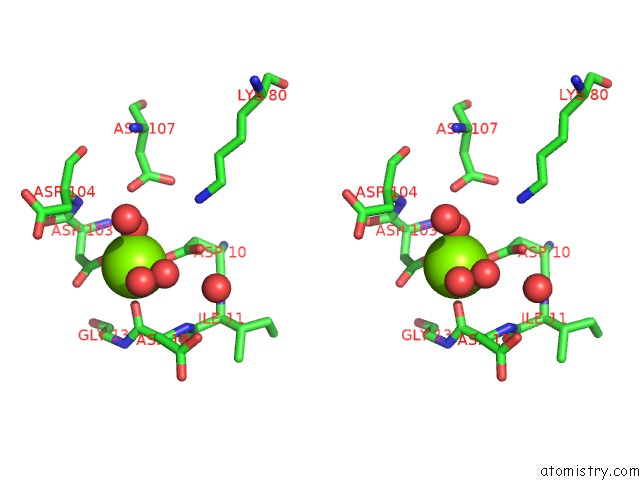
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
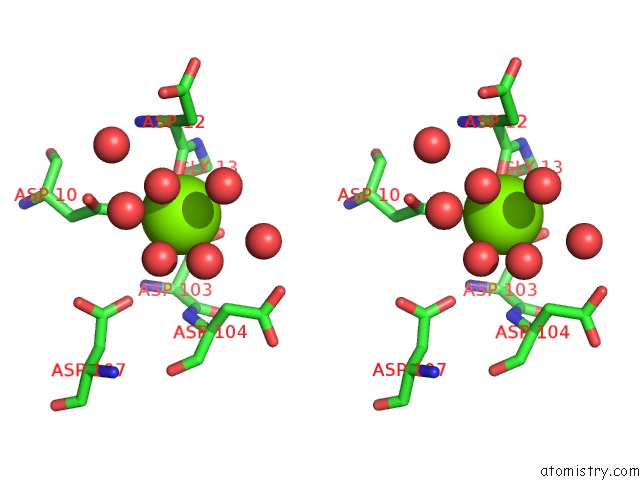
Magnesium binding site 4 out of 8 in 4hgq
Go back to
Magnesium binding site 4 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
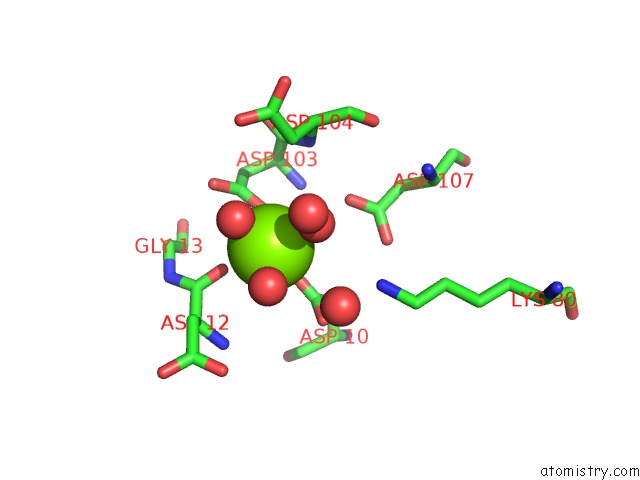
Mono view
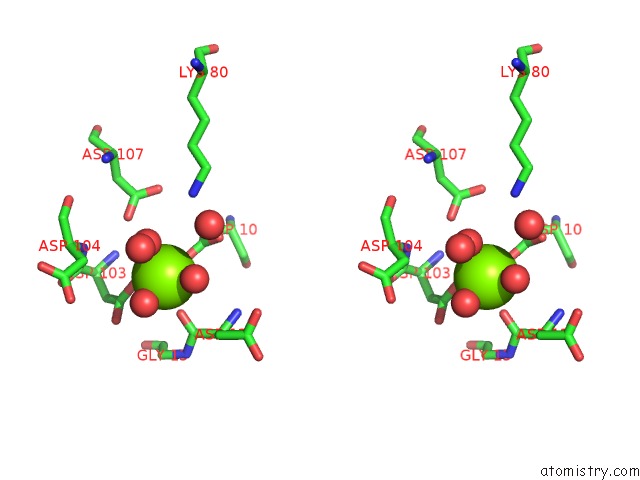
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
Magnesium binding site 5 out of 8 in 4hgq
Go back to
Magnesium binding site 5 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
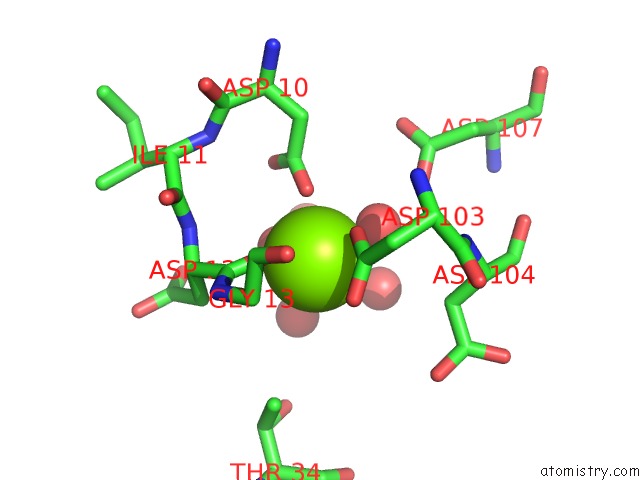
Mono view
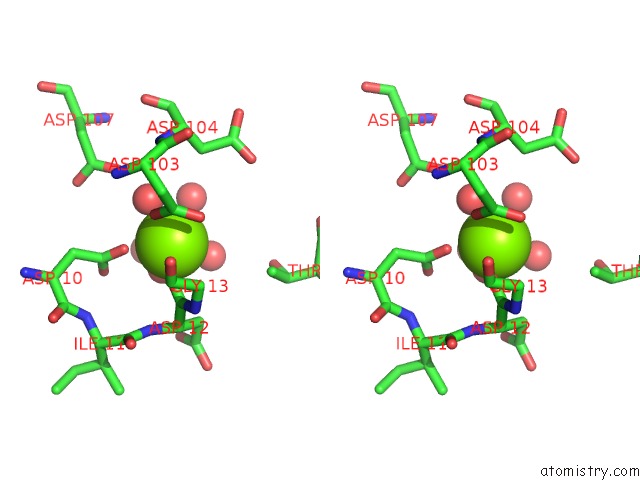
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
Magnesium binding site 6 out of 8 in 4hgq
Go back to
Magnesium binding site 6 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
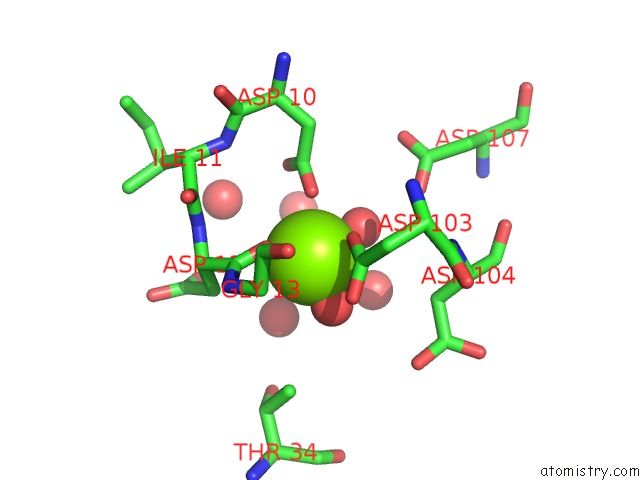
Mono view
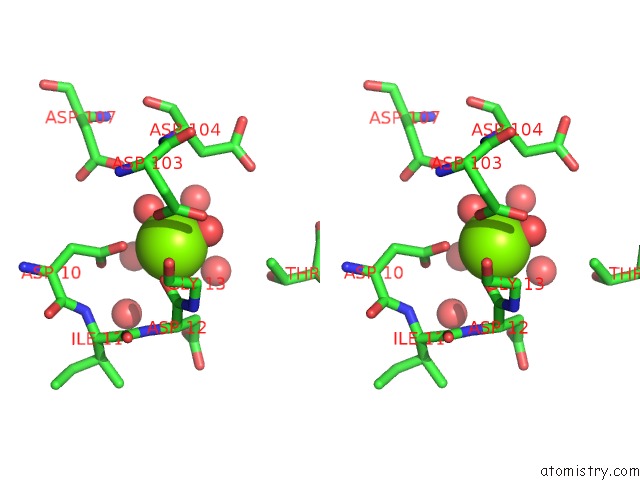
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
Magnesium binding site 7 out of 8 in 4hgq
Go back to
Magnesium binding site 7 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
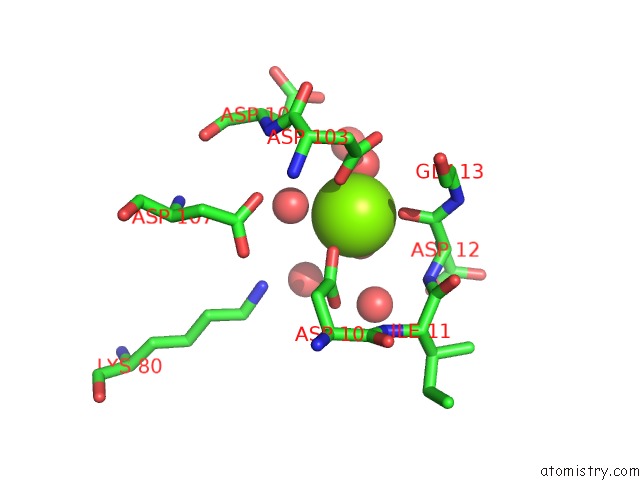
Mono view
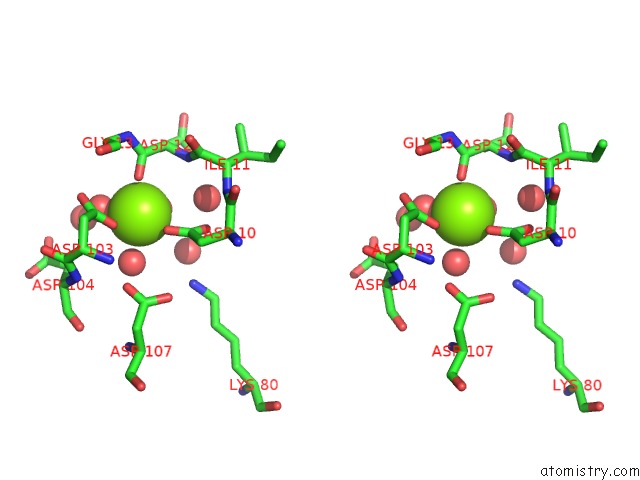
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
Magnesium binding site 8 out of 8 in 4hgq
Go back to
Magnesium binding site 8 out
of 8 in the Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron
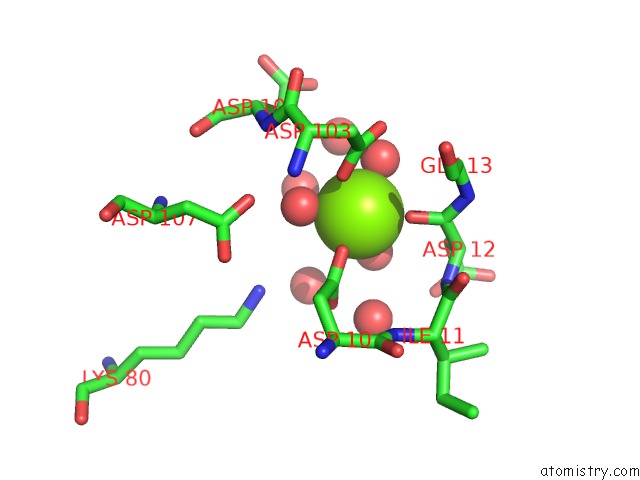
Mono view
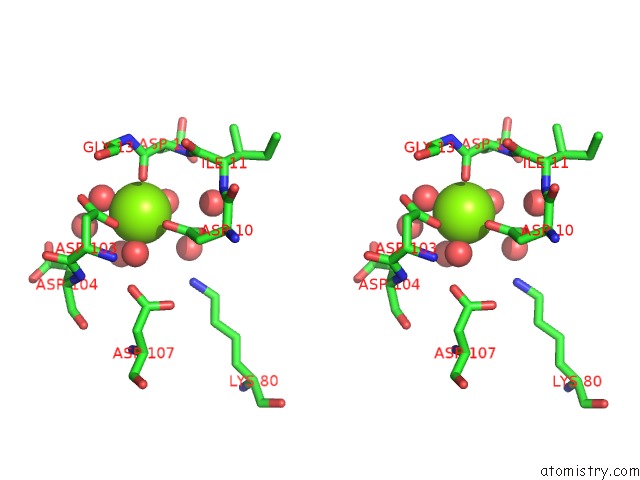
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Crystal Structure of E56A Mutant of 2-Keto-3-Deoxy-D-Glycero-D- Galactonononate-9-Phosphate Phosphohydrolase From Bacteroides Thetaiotaomicron within 5.0Å range:
|
Reference:
K.D.Daughtry,
H.Huang,
V.Malashkevich,
Y.Patskovsky,
W.Liu,
U.Ramagopal,
J.M.Sauder,
S.K.Burley,
S.C.Almo,
D.Dunaway-Mariano,
K.N.Allen.
Structural Basis For the Divergence of Substrate Specificity and Biological Function Within Had Phosphatases in Lipopolysaccharide and Sialic Acid Biosynthesis. Biochemistry V. 52 5372 2013.
ISSN: ISSN 0006-2960
PubMed: 23848398
DOI: 10.1021/BI400659K
Page generated: Mon Aug 11 13:50:51 2025
ISSN: ISSN 0006-2960
PubMed: 23848398
DOI: 10.1021/BI400659K
Last articles
Mg in 4JI1Mg in 4JI0
Mg in 4JI2
Mg in 4JI3
Mg in 4JHD
Mg in 4JH6
Mg in 4JH8
Mg in 4JH7
Mg in 4JH3
Mg in 4JH5